Chiral pool synthesis of enantiomerically pure morphan derivatives with κ receptor affinity from (S)-perillaldehyde
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ethylenediamines represent a promising class of κ receptor agonists. Recently, the first ethylenediamine – morphan hybrid 4 with high κ receptor affinity, but poor selectivity over the σ1 receptor was reported. Herein, the chiral pool synthesis of substituted morphans 19 and 29 is reported combining the morphan scaffold with the κ-pharmacophoric elements dichlorophenylacetamide and pyrrolidine. Starting from methyl (S)-perillate (12), a two-step sequence, i.e., epoxidation followed by reaction with benzylamine, provided enantiomerically pure morphan-8-carboxylate 10a. Functional group modifications of the ester moiety and the benzylamine substructure of 10a led to the pyrrolidinomethyl derivative 19. Key step of the synthesis of pyrrolidine 29 was a Curtius rearrangement, in which the intermediate isocyanate was trapped with benzyl alcohol to obtain the carbamate 27. The κ affinity of pyrrolidine 29 (Ki(κ) = 138 nM) was approximately 7-8-fold lower than the κ affinity of morphan 4 without further substituents. However, taking lipophilicity into account resulted in almost identical LLE values for 4 (LLE = 5.66) and 29 (LLE = 5.56). The additional OH moiety of 29 not only increased the polarity, but also the receptor selectivity, as 29 did no longer interact with σ1 receptors. Docking studies demonstrated similar binding poses of 4 and 29 at the κ receptor. Moreover, the reduced affinity of 29 towards κ and σ1 receptors could be explained. In vitro, 29 revealed high metabolic stability. Human peripheral blood mononuclear cells stimulated with lipopolysaccharide were used to show the anti-inflammatory and immunomodulatory effects of the κ receptor agonists 4 and 29.
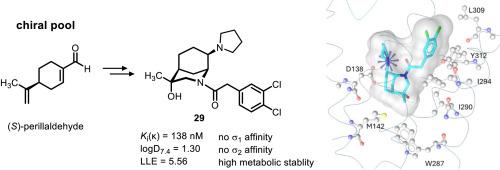
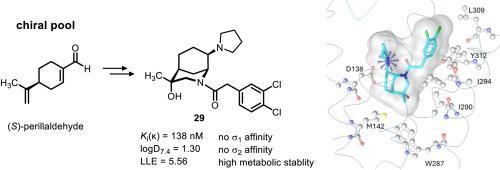
(S)-紫苏醛手性池合成具有κ受体亲和力的对映体纯morphan衍生物
乙二胺是一类很有前途的κ受体激动剂。近年来,首次报道了具有高κ受体亲和力,但对σ1受体选择性较差的乙二胺-吗啡杂合物4。本文报道了将morphan支架与κ-药效元素二氯苯乙酰胺和吡咯烷结合的手性池合成取代的morphan 19和29。从甲基(S)-紫苏酸酯(12)开始,经过两步的顺序,即环氧化,然后与苄胺反应,得到了对映体纯的morphan-8-羧酸10a。10a的酯部分和苯胺亚结构的官能团修饰导致了吡咯烷二甲基衍生物19。吡咯烷29合成的关键步骤是Curtius重排,中间的异氰酸酯被苯甲醇捕获,得到氨基甲酸酯27。pyrrolidine 29 (Ki(κ) = 138 nM)的κ亲和力比没有取代基的morphan 4的κ亲和力低约7-8倍。然而,考虑到亲脂性,LLE值为4 (LLE = 5.66)和29 (LLE = 5.56)几乎相同。由于29不再与σ1受体相互作用,增加的OH片段不仅提高了极性,而且提高了受体的选择性。对接研究显示在κ受体上有相似的4和29的结合姿势。此外,29对κ和σ1受体的亲和力降低也可以解释。在体外,29种显示出高代谢稳定性。用脂多糖刺激人外周血单个核细胞,观察κ受体激动剂4和29的抗炎和免疫调节作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


