Advances in sonodynamic therapy of multifunctional nanomaterials for bacterial infectious diseases
IF 23.5
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Bacterial infectious diseases are in urgent need of novel therapeutic strategies due to increased drug resistance and complex host-pathogen interaction mechanisms. This review systematically summarizes recent advances in sonodynamic therapy (SDT) utilizing multifunctional nanomaterials for the treatment of bacterial infections. The design and functionality of these nanomaterials predominantly rely on principles of coordination chemistry. Beginning with an analysis of pathogenesis, this article examines the dynamic interplay between bacterial virulence factors and host immune responses, as well as the evolution of drug resistance and patterns of bacterial colonization and dissemination. Furthermore, it elaborates on the mechanisms by which SDT induces synergistic structural damage to bacteria through cavitation-generated sonomechanical, sonochemical, and sonothermal effects. For six types of clinically refractory infections, such as myositis and osteomyelitis, nanomaterials can significantly improve the therapeutic effect through targeted delivery, enhanced stability of sonosensitizers and local acoustic field response. Combining photodynamic, photothermal, herbal active ingredients and immunotherapy further revealed the potential of SDT to synergize multiple therapies in bactericidal and host microenvironment modulation. Although SDT has demonstrated considerable advancements in antimicrobial treatment, several significant challenges remain. These include concerns regarding the biocompatibility and long-term safety of nanomaterials, the absence of standardized clinical protocols, the insufficient development of integrated theranostic platforms, and uncertainties surrounding the clinical safety and efficacy of current treatment strategies. Future research efforts should prioritize the design and synthesis of novel sonosensitizers, the refinement of ultrasound parameters, and the advancement of multimodal imaging-guided combination therapies based on SDT. Furthermore, rigorous evaluation of treatment safety and efficacy through well-designed large-animal infection models is essential to accelerate the clinical translation of SDT. Such developments are critical for addressing the growing need for precise therapeutic strategies against bacterial infections in the post-antibiotic era.

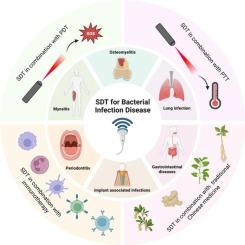
多功能纳米材料声动力治疗细菌感染性疾病的研究进展
由于细菌感染性疾病的耐药性增加和宿主-病原体相互作用机制复杂,迫切需要新的治疗策略。本文系统总结了利用多功能纳米材料进行声动力治疗(SDT)治疗细菌感染的最新进展。这些纳米材料的设计和功能主要依赖于配位化学原理。本文从致病机制的分析开始,探讨了细菌毒力因子与宿主免疫反应之间的动态相互作用,以及耐药性的演变和细菌定植和传播的模式。此外,它详细阐述了SDT通过空化产生的声力学、声化学和声温效应诱导细菌协同结构损伤的机制。对于肌炎、骨髓炎等6种临床难治性感染,纳米材料可通过靶向递送、增强声敏剂稳定性和局部声场反应等方式显著提高治疗效果。结合光动力、光热、草药活性成分和免疫治疗,进一步揭示了SDT在杀菌和宿主微环境调节方面协同多种治疗的潜力。尽管SDT在抗菌治疗方面取得了相当大的进展,但仍存在一些重大挑战。这些问题包括对纳米材料的生物相容性和长期安全性的担忧,缺乏标准化的临床方案,综合治疗平台的发展不足,以及当前治疗策略的临床安全性和有效性的不确定性。未来的研究工作应优先考虑新型声敏剂的设计和合成,超声参数的改进,以及基于SDT的多模态成像引导联合治疗的进展。此外,通过精心设计的大型动物感染模型来严格评估治疗的安全性和有效性对于加速SDT的临床转化至关重要。这些发展对于解决后抗生素时代对细菌感染的精确治疗策略日益增长的需求至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


