Dynamic Site Induced Self-Adaptive Adsorption on Bifunctional Catalysts for Highly Selective Oxidative Switching from Urea-to-Water Electrolysis
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The adsorption strength of hydroxide ions affects the catalytic activities of the oxygen evolution reaction (OER) and urea oxidation reaction (UOR). Inspired by the “for” loop function in computer programming, this work proposes a dynamic active site strategy to modulate “self-adaptive” adsorption of OH− to achieve a continuous urea-to-water electrolysis for the first time, with a highly selective switching from UOR to OER. Specifically, MoO42−-doped NiCo-based layered double hydroxide (NiCo-LDH-MO) is developed as the UOR/OER bifunctional catalyst through the hydrothermal doping and electrochemical reconstruction of Prussian blue analogs. With the aid of density functional theory (DFT) calculation and the in situ characterizations, it suggests that OH− is preferentially adsorbed on Co sites to generate CoOOH for UOR in the presence of urea, and then self-adaptively adsorbs on Ni sites to generate NiOOH for OER after the removal of urea. As a result, the reconstructed electrode exhibits remarkably low potentials of 1.27/1.36 V for UOR at 10/100 mA cm−2, respectively, and only 347 mV overpotential for OER at 100 mA cm−2. This work demonstrates a new pathway for the effective hydrogen and oxygen production from urea-contaminated water, such as human urine and industrial wastewater.
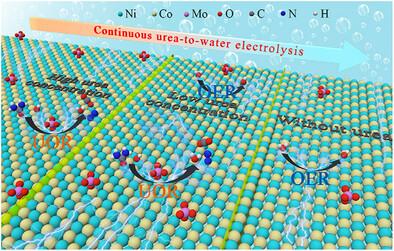
双功能催化剂的动态位点诱导自适应吸附在尿素-水电解中的高选择性氧化转换
氢氧根离子的吸附强度影响析氧反应(OER)和尿素氧化反应(UOR)的催化活性。受计算机编程中的“for”循环功能的启发,本研究首次提出了一种动态活性位点策略来调节OH -的“自适应”吸附,以实现从UOR到OER的高度选择性切换,实现连续的尿素-水电解。具体而言,通过水热掺杂和普鲁士蓝类似物的电化学重构,开发了MoO42−掺杂镍基层状双氢氧化物(NiCo-LDH-MO)作为UOR/OER双功能催化剂。通过密度泛函理论(DFT)计算和原位表征表明,在尿素存在的情况下,OH -优先吸附在Co位点生成用于UOR的CoOOH,然后在去除尿素后自适应吸附在Ni位点生成用于OER的NiOOH。结果表明,重构电极在10/100 mA cm - 2时UOR的过电位分别为1.27/1.36 V,而在100 mA cm - 2时OER的过电位仅为347 mV。这项工作为从人类尿液和工业废水等被尿素污染的水中有效生产氢和氧提供了一条新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


