Sialylated Macrophage Nano-Decoys Mitigate Inflammatory-ROS Microenvironment and Reprogram Endothelial Function in Myocardial Infarction
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Myocardial infarction (MI) induces the infiltration of abundant immune cells, such as macrophages, and thereby elevated inflammation characterized by elevated reactive oxygen species (ROS), which leads to dysfunction of myocardial microvessels and exacerbates myocardial ischemia and necrosis. Intravenously injected drugs hardly remain in the MI region of the heart, whereas hydrogels or cardiac patches need a complicated thoracotomy. In this study, a ROS-scavenging polyurethane nano-decoys (NDs) modified with N-acetylneuraminic acid (sialic acid, SA) (PTSA) is developed for therapy of MI via convenient intravenous injection. Due to the high affinity of SA to macrophages, PTSA is better camouflaged to avoid fast immune clearance, allowing long-term retention in the damaged myocardium. The loaded nicorandil (NIC) could be faster released in response to ROS environment, which in turn scavenged ROS and alleviated the inflammation. The NIC@PTSA effectively inhibited abnormal mitochondrial function and apoptosis of cardiomyocytes in vitro. Under the synergistic effect of ROS-scavenging and NIC release, the function of endothelial cells is reprogrammed, promoting the process of vascularization. Treatment of NIC@PTSA in vivo significantly reduced oxidative stress, promoted angiogenesis, and inhibited adverse ventricular remodeling. These multifunctional NDs provide an effective strategy for MI therapy, especially from the viewpoint of realistic application.
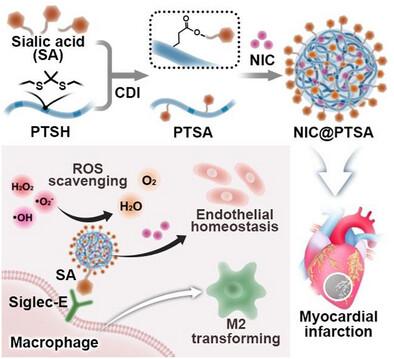
唾液化巨噬细胞纳米诱饵减轻炎症- ros微环境和心肌梗死内皮功能重编程
心肌梗死(MI)诱导巨噬细胞等大量免疫细胞浸润,从而引起以活性氧(ROS)升高为特征的炎症升高,导致心肌微血管功能障碍,加剧心肌缺血坏死。静脉注射药物很难留在心脏的心肌区,而水凝胶或心脏贴片则需要复杂的开胸手术。本研究开发了一种n -乙酰神经氨酸(唾液酸,SA) (PTSA)修饰的清除ros的聚氨酯纳米诱饵(ndds),通过方便的静脉注射治疗心肌梗死。由于SA对巨噬细胞的高亲和力,PTSA被更好地伪装以避免快速免疫清除,允许长期保留在受损的心肌中。负载的尼可地尔(nicorandil, NIC)能在ROS环境下快速释放,从而清除ROS,减轻炎症。NIC@PTSA能有效抑制心肌细胞线粒体功能异常和凋亡。在ros清除和NIC释放的协同作用下,内皮细胞的功能被重新编程,促进血管化进程。NIC@PTSA在体内治疗可显著降低氧化应激,促进血管生成,抑制不利的心室重构。这些多功能NDs为心肌梗死治疗提供了一种有效的策略,特别是从现实应用的角度来看。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


