Environmental Control of Regio-/Stereoselectivity Across Mechanistically Similar Transformations
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The control of regio- and stereoselectivity in organic transformations remains a foundational challenge in synthetic chemistry. While chemoselectivity can often be influenced by careful selection of reagents or protective groups, controlling regio- and stereoselectivity is far more nuanced, often requiring highly specific reaction conditions. In this work, a unified approach for controlling both regio- and stereoselectivity across mechanistically related transformations is explored, specifically focusing on epoxide openings and carbonyl additions. It is demonstrated that the same environmental factors, here the nature of the Lewis acid (LA), can be leveraged to influence divergent outcomes in these two reaction types by amplifying inherent differences between reaction sites/faces, leading to highly selective transformations. The systematic evaluation of over 30 Lewis acids revealed a dichotomy between “labile” and “strong” activation modes, where strong LAs, such as AlCl3 and SnCl4, drive the highest levels of regio- and diastereoselectivity for both transformations. Further, there exists nuanced differences between the degree of influence of some LA within these mechanistically related transformations. These findings suggest that environmental factors can be broadly applied across different mechanistic classes to achieve selectivity, offering a versatile strategy for reaction optimization.
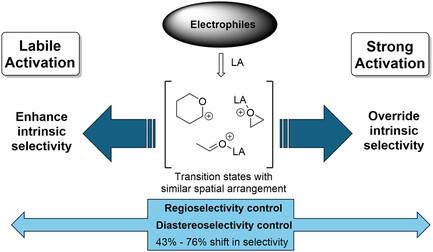
机制相似转化中区域/立体选择性的环境控制
有机转化中区域选择性和立体选择性的控制仍然是合成化学中的一个基本挑战。虽然化学选择性通常会受到仔细选择试剂或保护基团的影响,但控制区域和立体选择性则要细致得多,通常需要高度特定的反应条件。在这项工作中,我们探索了一种统一的方法来控制区域选择性和立体选择性,特别是环氧化物开孔和羰基加成。研究表明,相同的环境因素,即刘易斯酸(LA)的性质,可以通过放大反应位点/面之间的固有差异来影响这两种反应类型的不同结果,从而导致高度选择性的转化。对30多种路易斯酸的系统评价揭示了“不稳定”和“强”活化模式之间的两分法,其中强LAs,如AlCl3和SnCl4,对这两种转化都具有最高的区域选择性和非对映选择性。此外,在这些机制相关的转变中,一些LA的影响程度存在细微差别。这些发现表明,环境因素可以广泛应用于不同的机制类别,以实现选择性,为反应优化提供了一个通用的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


