Development of in vitro-in vivo correlation for establishing patient-centric quality standards of dissolution for lamotrigine extended-release tablets
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-23
DOI:10.1016/j.ejpb.2025.114874
引用次数: 0
Abstract
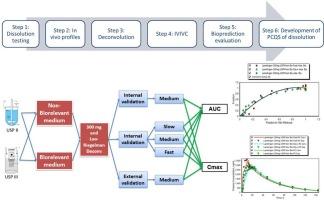
In vitro-in vivo correlation (IVIVC) studies have been commonly used for assessing the impact of formulation and manufacturing changes on drug performance. The current study developed an IVIVC to establish patient-centric quality standards (PCQS) for dissolution using lamotrigine extended release (ER) 300 mg tablets as model formulation. Dissolution of Lamotrigine ER tablets was tested using various dissolution apparatus (USP II & III), dissolution media (biorelevant, non-bio relevant), media composition, pH, and hydrodynamics. The plasma lamotrigine concentration time profiles following oral administration were simulated using a physiologically based pharmacokinetic (PBPK) model. This PBPK model was developed and verified using plasma lamotrigine profiles following administration of lamotrigine intravenous (IV) solution and oral immediate-release (IR) tablets. Model verification results showed accurate prediction of Cmax and AUC following IV and IR lamotrigine administration with confidence level exceeding 95 %. Various IVIVC models were investigated using dissolution data of fast, medium, and slow ER lamotrigine 300 mg tablets manufactured in-house. Optimal IVIVC models were obtained using a second order polynomial and a two-compartment Loo-Riegelman deconvolution. The results of IVIVC goodness of fit showed that the dissolution condition in standard compendial media using USP apparatus II established a Level A IVIVC. This IVIVC model passed both internal and external validation criteria. Using these dissolution conditions, a PCQS of ≤10 % release at 2 h, ≤45 % at 6 h, and ≥80 % at 18 h was derived. In conclusion, this study demonstrates that a PCQS for lamotrigine ER tablets dissolution can be established using verified PBPK and validated IVIVC model and offers a reliable approach for assessment of product performance.
建立以患者为中心的拉莫三嗪缓释片溶出度质量标准的体内外相关性研究进展。
体外体内相关性(IVIVC)研究通常用于评估配方和制造变化对药物性能的影响。本研究以拉莫三嗪缓释片(ER) 300 mg为模型制剂,建立以患者为中心的溶出度质量标准(PCQS)。采用各种溶出仪(USP II和III)、溶出介质(生物相关的、非生物相关的)、介质组成、pH值和流体动力学测试拉莫三嗪ER片的溶出度。采用基于生理的药代动力学(PBPK)模型模拟口服给药后血浆拉莫三嗪浓度时间曲线。采用拉莫三嗪静脉(IV)溶液和口服即刻释放(IR)片剂后血浆拉莫三嗪谱建立并验证了PBPK模型。模型验证结果显示,IV和IR给药后的Cmax和AUC预测准确,置信水平超过95 %。采用国产快速、中速、慢速拉莫三嗪300 mg片的溶出度数据,研究了各种IVIVC模型。利用二阶多项式和双室Loo-Riegelman反卷积获得了最优的IVIVC模型。IVIVC拟合优度结果表明,使用USP仪器II在标准药典介质中的溶出条件建立a级IVIVC。该IVIVC模型通过了内部和外部验证标准。在此条件下,得到了2 h时释放量 ≤10 %,6 h时释放量≤45 %,18 h时释放量 ≥80 %的PCQS。综上所述,本研究可以利用验证的PBPK和验证的IVIVC模型建立拉莫三嗪ER片溶出度的PCQS,为评价产品性能提供了可靠的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


