Micellar-Assisted Oxidation of D-Glucose by Imidazolium Fluorochromate in a 50% (v/v) Aqueous Acetic Acid Medium: A Kinetic and Mechanistic Approach
Abstract
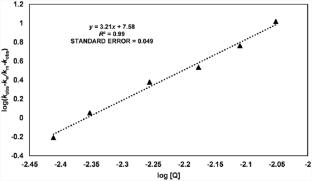
This work examined the impact of sodium dodecyl sulfate (SDS) surfactant on the oxidation kinetics of D-glucose employing imidazolium fluorochromate (IFC) as the oxidant. The experiments were conducted under pseudo-first-order conditions at 30 °C, with D-glucose in a significantly higher concentration than IFC. The solvent medium used was 50% (v/v) aqueous acetic acid. The reaction demonstrated a first-order dependence on [IFC] and [HClO4]. The order for [D-glucose] oxidation kinetics was slightly less than one. Perchloric acid acted as a catalyst in this process. The addition of acrylonitrile had no effect on rate constants (kobs). kobs decreased with an increase in the concentration of Mn(II) ions. An increase in the dielectric constant of the medium had an inverse effect on kobs. The initial increase in [SDS] led to a corresponding rise in kobs, though a saturation in kobs was observed for elevated [SDS]. The Menger-Portnoy and Piszkiewicz models were used to quantitatively assess the experimental results. The binding constant (Kp), cooperativity index (n), and various thermodynamic parameters in the presence of SDS were calculated. Infrared and mass spectroscopy were used to ascertain D-gluconolactone as the primary product of the reaction. Based on these findings, a reaction scheme has been suggested.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


