Influence of Anode Reactivity and Chemical Crossover on the Formation of Cathode-Electrolyte Interphase in High-Nickel Layered Oxide Cathodes
IF 26
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
As the push for lithium-ion batteries (LIBs) with high-energy density grows, systems pairing high-nickel cathodes with high-capacity anodes have become attractive; however, these electrodes individually suffer from high surface reactivities, leading to interfacial instabilities. When paired together, further issues arise, with cathode-to-anode crossover being a well-known phenomenon. In contrast, anode-to-cathode crossover remains underexplored, especially in systems that undergo large volume changes. Here, a comparison of the influence of anode reactivity on cathode surface degradation is presented by pairing LiNi0.8Mn0.1Co0.1O2 (NMC811) cathode with graphite, prelithiated silicon suboxide (SiOx), and lithium-metal anodes. Voltage curves and differential capacity analysis show that all cells experience polarization growth throughout cycling. A combination of electrochemical techniques, such as operando galvanostatic electrochemical impedance spectroscopy (GEIS), and surface analyses, such as scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS), reveal that cycling against more reactive anodes promotes the formation of a thicker, organic-rich cathode electrolyte interphase (CEI), which suffers from impedance growth and large irreversible capacity loss. Post-mortem characterization with XPS and SEM confirms compositional and morphological changes at the cathode surface and the cycled separator. The findings provide insights into the role of anode-driven degradation of high-Ni cathodes, promoting further understanding of two-way crossover in LIBs.
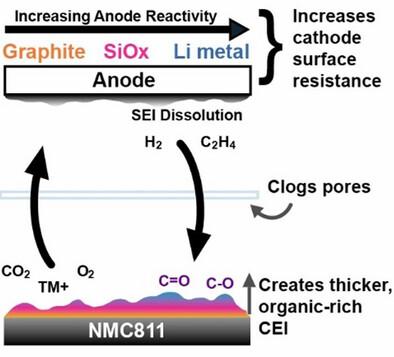
阳极反应性和化学交叉对高镍层状氧化物阴极阴极-电解质界面形成的影响
随着对高能量密度锂离子电池(LIBs)的推动,高镍阴极与高容量阳极配对的系统变得有吸引力;然而,这些电极单独受到高表面反应性的影响,导致界面不稳定。当配对在一起时,进一步的问题出现了,阴极到阳极交叉是一个众所周知的现象。相比之下,阳极-阴极交叉仍然没有得到充分的研究,特别是在经历大体积变化的系统中。本文通过将LiNi0.8Mn0.1Co0.1O2 (NMC811)阴极与石墨、预锂化亚氧化硅(SiOx)和锂金属阳极配对,比较了阳极反应性对阴极表面降解的影响。电压曲线和差分容量分析表明,在整个循环过程中,所有电池都经历了极化生长。电化学技术(如operando恒流电化学阻抗谱(GEIS))和表面分析(如扫描电镜(SEM)和x射线光电子能谱(XPS))的结合表明,在反应性更强的阳极上循环促进了更厚、富有机物的阴极电解质界面(CEI)的形成,该界面受到阻抗增长和大的不可逆容量损失的影响。用XPS和SEM进行尸检,证实了阴极表面和循环分离器的成分和形态变化。这些发现为高镍阴极的阳极驱动降解的作用提供了见解,促进了对锂离子电池双向交叉的进一步理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


