Nickel-Catalyzed Cross Coupling of N-Alkenyl Heterocycles and Aryl Sulfonium Salts: Expedient Access to N-Benzyl Heterocycles
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
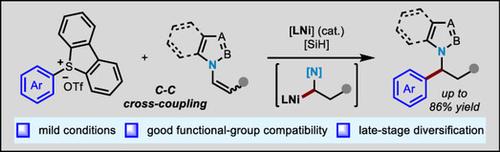
A nickel-catalyzed system is developed that enables the coupling between aryldibenzothiophenium salts and N-alkenyl heterocycles. Traditionally, medicinally relevant N-benzylic heterocycles are constructed via harsh substitution conditions between the N-H heterocycle and a benzyl electrophile in the presence of strong bases. The CC cross coupling strategy presents attractive potential for the synthesis of these important scaffolds, e.g., carboetomidate. Herein, it is documented that the combination of a site-selective CH dibenzothiophenation with a nickel-catalyzed C(sp3)–C(sp2) cross coupling grants access to a diverse range of synthetically useful N-benzylic heterocycles, i.e., alkylated arenes. In particular, the mild conditions and good functional group compatibility permit the rapid assembly of molecular complexity and late-stage functionalization of bioactive compounds, such as clofibrate, fenofibrate, and fenbufen, thus providing an orthogonal protocol to existing SN2 and CN coupling approaches.
镍催化n -烯基杂环与芳基磺酸盐的交叉偶联:n -苄基杂环的便捷途径
开发了一种镍催化体系,使芳基二苯并噻吩盐与n -烯基杂环之间的偶联成为可能。传统上,医学上相关的n -苯基杂环是在强碱存在下,通过N-H杂环和苯基亲电试剂之间的苛刻取代条件构建的。C - _ - C交叉偶联策略为这些重要支架的合成提供了诱人的潜力,例如,碳托咪酯。本文记录了位点选择性C - H二苯并噻吩与镍催化的C(sp3) -C (sp2)交叉偶联的结合,可以获得多种合成有用的n -苯基杂环,即烷基化芳烃。特别是,温和的条件和良好的官能基团相容性允许分子复杂性的快速组装和生物活性化合物的后期功能化,如氯贝特、非诺贝特和芬布芬,从而为现有的SN2和C -氨基偶联方法提供了一个正交方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


