Locally constrained xylene-based cyclic mimetics of SOCS3 protein
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The development of SOCS3 peptidomimetics targeting the JAK2 signaling pathway presents a promising strategy for modulating cytokine-driven diseases. In this study, we designed four novel monocyclic analogues of a previously reported linear lead compound (KIRCONG chim PEG), introducing local conformational constraints via regioselective thioether cyclization of either the KIR (thio-monoleft) or CONG (thio-monoright) regions. Each variant was synthesized with one or two PEG moieties (PEG1 or PEG2) to modulate solubility and flexibility. Microscale Thermophoresis (MST) revealed that thio-monoright PEG1 exhibited the highest affinity for the JAK2 catalytic domain, and inhibition assays further confirmed its superior ability to suppress JAK2 mediated phosphorylation. Circular dichroism (CD) and metadynamics simulations indicated enhanced structural organization and intramolecular hydrogen bonding in cyclic analogues, especially thio-monoright PEG1, as supported by Natural Bond Orbital (NBO) and Second Order Perturbation Theory (SOPT) analyses. Conversely, PEG2 analogues showed reduced activity and solubility, likely due to increased flexibility and aggregation arising from hydrophobic surface exposure, as demonstrated by fluorescence spectroscopy and solvent-accessibility calculations. Overall, thio-monoright PEG1 represents a promising conformationally stabilized SOCS3 mimetic with improved bioactivity, providing a rational foundation for further optimization of JAK2 targeting peptide therapeutics.
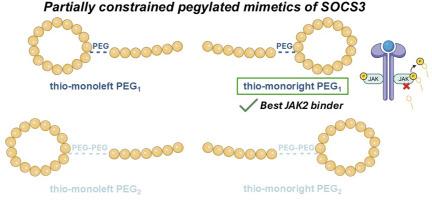
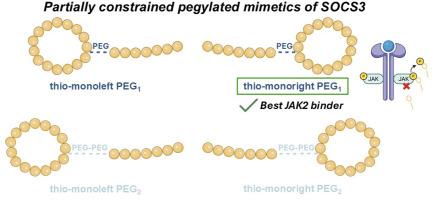
SOCS3蛋白的局部约束二甲苯环模拟物
靶向JAK2信号通路的SOCS3肽模拟物的发展为调节细胞因子驱动的疾病提供了一种有前途的策略。在这项研究中,我们设计了四种新的单环类似物,用于先前报道的线性先导化合物(KIRCONG chim PEG),通过区域选择性硫醚环化KIR(硫基单左)或CONG(硫基单右)区域引入局部构象约束。每个变体由一个或两个PEG片段(PEG1或PEG2)合成,以调节溶解度和柔韧性。微尺度热电泳(MST)显示,硫基单右PEG1对JAK2催化结构域具有最高的亲和力,抑制实验进一步证实了其抑制JAK2介导的磷酸化的优越能力。圆二色性和元动力学模拟表明,环类似物的结构组织和分子内氢键增强,特别是硫基单链PEG1,自然键轨道(NBO)和二阶摄动理论(SOPT)分析支持了这一结果。相反,正如荧光光谱和溶剂接近性计算所证明的那样,peg2类似物的活性和溶解度降低,可能是由于疏水表面暴露增加了灵活性和聚集性。综上所述,硫代单权PEG1代表了一种有前景的构象稳定的SOCS3模拟物,具有更高的生物活性,为进一步优化jak2靶向肽疗法提供了合理的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


