HSP90 Mediates Targeted Degradation of Nonclient Protein PARP1 for Breast Cancer Treatment
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Heat shock protein 90 (HSP90) has been developed as an effector in mediating targeted protein degradation (TPD), representing a novel strategy in TPD drug design. The majority of reported cases of HSP90-mediated degradation targeted HSP90 client proteins, including BRD4-CHAMPs, CDK4/6-HEMTACs, and GPX4-HIM-PROTACs. However, HSP90 ATPase inhibitor was used to design the above molecules, which might cause nonspecific degradation of other client proteins. In this study, we sought to broaden the scope of HSP90-mediated proteolysis-targeting chimeras (HSPTACs) from client protein degradation to include nonclient protein degradation. Herein, we induced unnatural interactions between poly(ADP-ribose) polymerase-1 (PARP1), a nonclient protein of HSP90, and HSP90 by bridging them with a small molecule (DDO3602). DDO3602 effectively induced PARP1 degradation through a multi-E3 ubiquitin ligase-mediated degradation pathway. In general, this study demonstrates that DDO3602 can degrade the HSP90 nonclient protein PARP1 through the ubiquitin-proteasome pathway and exhibits tumor-selective pharmacokinetics.
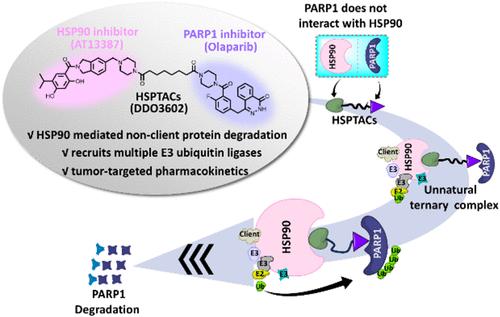
HSP90介导非客户蛋白PARP1靶向降解用于乳腺癌治疗
热休克蛋白90 (Heat shock protein 90, HSP90)作为靶蛋白降解(targeted protein degradation, TPD)的一种效应体,代表了一种新的TPD药物设计策略。大多数报道的HSP90介导的降解针对HSP90客户蛋白,包括BRD4-CHAMPs, CDK4/6-HEMTACs和gpx4 - hm - protacs。然而,使用HSP90 atp酶抑制剂设计上述分子,可能会导致其他客户蛋白的非特异性降解。在这项研究中,我们试图将hsp90介导的靶向蛋白水解嵌合体(HSPTACs)的范围从客户蛋白降解扩大到包括非客户蛋白降解。在这里,我们通过用一个小分子(DDO3602)桥接HSP90的非客户蛋白聚(adp -核糖)聚合酶-1 (PARP1)诱导它们之间的非自然相互作用。DDO3602通过多e3泛素连接酶介导的降解途径有效诱导PARP1降解。总的来说,本研究表明DDO3602可以通过泛素-蛋白酶体途径降解HSP90非客户蛋白PARP1,并表现出肿瘤选择性药代动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


