Beyond Conventional Methods for Evaluating Charge Transfer Kinetics in Aqueous Zinc-Ion Batteries: Insights from Ultramicroelectrode Voltammetry and Marcus–Hush Theory
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
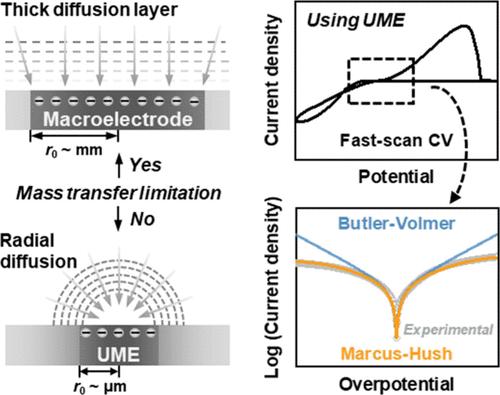
Understanding intrinsic charge transfer kinetics is essential for aqueous zinc-ion batteries (AZIBs). In this work, we employ fast-scan cyclic voltammetry with ultramicroelectrodes (UMEs) to eliminate mass transfer limitations and accurately extract the exchange current density (j0) and reorganization energy (λ) in representative zinc electrolytes: Zn(ClO4)2, ZnSO4, Zn(TfO)2, and ZnCl2. While the Butler–Volmer model accurately describes kinetics at low overpotentials, it fails to capture the nonlinear Tafel behavior at higher overpotentials. In contrast, the Marcus–Hush model successfully accounts for these deviations while also providing physically meaningful kinetic parameters across a wider potential range. Additionally, electrolytes with larger anions and higher viscosities exhibit lower values for j0 and higher values for λ, indicating that solvation structure and ion–solvent interactions contribute to interfacial kinetics. These findings highlight the limitations of the Butler–Volmer model and demonstrate that Marcus–Hush theory offers a more rigorous and accurate approach for evaluating charge transfer in AZIBs.
超越传统方法评估电荷转移动力学在水锌离子电池:洞察从超微电极伏安法和马库斯- hush理论
了解本征电荷转移动力学对水锌离子电池(azib)至关重要。在这项工作中,我们利用超微电极(UMEs)快速扫描循环伏安法消除了传质限制,准确地提取了代表性锌电解质:Zn(ClO4)2、ZnSO4、Zn(TfO)2和ZnCl2中的交换电流密度(j0)和重组能(λ)。虽然Butler-Volmer模型准确地描述了低过电位下的动力学,但它无法捕获高过电位下的非线性塔菲尔行为。相比之下,Marcus-Hush模型成功地解释了这些偏差,同时还在更大的势能范围内提供了有物理意义的动力学参数。此外,阴离子较大、粘度较高的电解质表现出较低的j0值和较高的λ值,表明溶剂化结构和离子-溶剂相互作用有助于界面动力学。这些发现突出了Butler-Volmer模型的局限性,并证明了Marcus-Hush理论为评估azib中的电荷转移提供了更严格和准确的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


