A bacterial signal coordinates plant-microbe fitness trade-off to enhance sulfur deficiency tolerance in plants
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Plant-associated microorganisms interact with each other and with host plants via intricate chemical signals, offering multiple benefits, including enhanced nutrition. We report a mechanism through which the rhizosphere microbiome improves plant growth under sulfur (S) deficiency. Disruption of plant S homeostasis caused a coordinated shift in the composition and S-metabolism of the rhizosphere microbiome. Leveraging this, we developed an 18-membered synthetic rhizosphere bacterial community (SynCom) that rescued the growth of Arabidopsis and a leafy Brassicaceae vegetable under S-deficiency. This beneficial trait is taxonomically widespread among SynCom members, with bacterial pairs providing both synergistic and neutral effects on host growth. Notably, stronger competitive interactions among SynCom members conferred greater fitness benefits to the host, suggesting a trans-kingdom (plant-microbe) fitness trade-off. Finally, guided chemical screening, deletion knockout mutants, and targeted metabolomics identified and validated microbially released glutathione (GSH) as the necessary bioactive signal that coordinates the trans-kingdom fitness trade-off and improves plant growth under sulfur limitation.
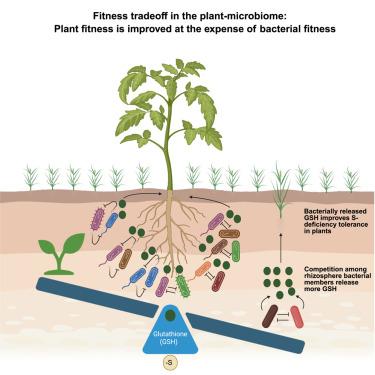
细菌信号协调植物与微生物的适应度权衡,以增强植物的硫缺乏耐受性
植物相关微生物通过复杂的化学信号相互作用,并与寄主植物相互作用,提供多种益处,包括增强营养。我们报告了一个机制,通过根际微生物组促进植物生长的硫(S)缺乏。植物S稳态的破坏引起根际微生物组组成和S代谢的协调变化。利用这一点,我们开发了一个由18个成员组成的合成根际细菌群落(SynCom),该群落在缺s条件下挽救了拟南芥和芸苔科绿叶蔬菜的生长。这种有益的性状在SynCom成员中广泛存在,细菌对对宿主生长既有协同作用,也有中性作用。值得注意的是,SynCom成员之间更强的竞争相互作用给宿主带来了更大的适应性利益,这表明存在跨界(植物-微生物)适应性权衡。最后,通过指导化学筛选、缺失敲除突变体和靶向代谢组学,鉴定并验证了微生物释放的谷胱甘肽(GSH)是协调跨界适应性权衡和改善硫限制下植物生长的必要生物活性信号。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


