CircCDYL promotes glycolysis to drive the progression of nasopharyngeal carcinoma
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
CircRNAs play critical roles in the onset and progression of nasopharyngeal carcinoma (NPC), although their underlying mechanisms remain incompletely understood.Objectives
In this study, we investigated the mechanism of circCDYL in promoting the proliferation, invasion and migration of NPC in vitro and in vivo.Methods
We reanalyzed RNA sequencing data (GSE137543, PRJNA391554) and validated findings with RT-qPCR and in situ hybridization experiments. Functional assays were subsequently performed both in vitro and in vivo.Results
We identified circCDYL as being highly expressed in NPC, with its expression levels positively correlated with clinical tumor staging. Functionally, circCDYL promotes tumor proliferation and metastasis both in vitro and in vivo. Mechanistically, circCDYL binds to the 3′-UTR of LDHA mRNA, stabilizing it and upregulating LDHA protein expression, thereby enhancing cellular glycolysis and increasing lactate production and accumulation. The elevated lactate, in turn, promotes lactylation of the actin-binding protein CFL1 at lysine 22. This modification reduces cell adhesion and stiffness, ultimately facilitating tumor cell proliferation, invasion, and migration.Conclusion
These findings indicate that circCDYL and its downstream pathways may serve as potential diagnostic markers or therapeutic targets for NPC.
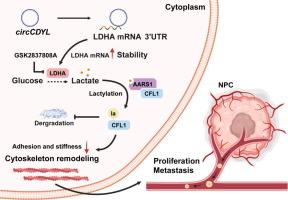
CircCDYL促进糖酵解,驱动鼻咽癌的进展
circrnas在鼻咽癌(NPC)的发生和发展中起着关键作用,尽管其潜在机制尚不完全清楚。目的研究circCDYL在体外和体内促进鼻咽癌细胞增殖、侵袭和迁移的作用机制。方法重新分析RNA测序数据(GSE137543, PRJNA391554),并采用RT-qPCR和原位杂交实验验证结果。随后进行了体外和体内功能测定。结果circCDYL在鼻咽癌中高表达,其表达水平与临床肿瘤分期呈正相关。在功能上,circCDYL在体内和体外都能促进肿瘤的增殖和转移。机制上,circCDYL结合LDHA mRNA的3 ' -UTR,稳定LDHA mRNA,上调LDHA蛋白表达,从而促进细胞糖酵解,增加乳酸的产生和积累。升高的乳酸反过来促进肌动蛋白结合蛋白CFL1在赖氨酸22处的乳酸化。这种修饰降低了细胞的粘附性和硬度,最终促进了肿瘤细胞的增殖、侵袭和迁移。结论circCDYL及其下游通路可作为鼻咽癌的潜在诊断标志物或治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


