Cs3Bi2Br9 QDs/BiOBr heterojunction photoelectrochemical biosensor with APE1 enzyme-driven bipedal DNA walker signal amplification for miRNA-320d detection
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
MicroRNAs (miRNAs), as pivotal biomarkers, demonstrate critical significance in early cancer diagnosis through their sensitive detection. An ultrasensitive photoelectrochemical (PEC) sensing platform for miRNA-320d detection was developed by integrating apurinic/apyrimidinic endonuclease 1 (APE1) enzyme-driven bipedal DNA walker amplification strategy.
Results
The platform employed a Cs3Bi2Br9 QDs/BiOBr Z-scheme heterojunction as the photoactive material, which generated a robust anodic photocurrent. Upon immobilizing alkaline phosphatase (ALP)-conjugated gold nanoparticles carrying apurine/pyrimidine (AP) site-modified L1 (ALP-Au NPs-L1) probes on the heterojunction surface, catalytic hairpin assembly (CHA)-generated 3D bipedal DNA walker walked in the presence of miRNA-320d, hybridizing with L1 to form duplex structures. The APE1 enzyme then selectively cleaved these duplexes, triggering the release of ALP-Au NPs from the electrode surface. This spatial separation deactivated the catalytic capacity of ALP, inducing a pronounced photocurrent attenuation. By synergizing the exceptional PEC performance of the Cs3Bi2Br9 QDs/BiOBr heterojunction, specific recognition and efficient cleavage of APE1 enzyme, and 3D walker-mediated signal amplification, this platform achieved ultrasensitive miRNA-320d detection with a detection limit of 0.1 fM and a linear range spanning 1 fM to 1 nM.
Significance
This study established novel conceptual frameworks for implementing emerging perovskite materials in PEC biosensing platforms targeting microRNA detection.
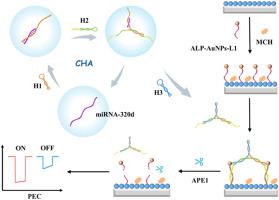
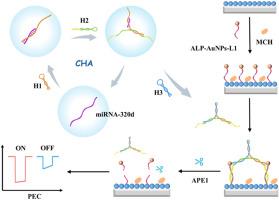
基于APE1酶驱动双足DNA Walker信号放大的Cs3Bi2Br9 QDs/BiOBr异质结光电化学生物传感器用于miRNA-320d检测
micrornas (miRNAs)作为关键的生物标志物,通过其灵敏的检测,在早期癌症诊断中具有重要意义。通过整合无尿嘧啶/无嘧啶内切酶1 (APE1)酶驱动双足DNA walker扩增策略,建立了一种检测miRNA-320d的超灵敏光电化学(PEC)传感平台。结果该平台采用了Cs3Bi2Br9 QDs/BiOBr Z-scheme异质结作为光活性材料,产生了强大的阳极光电流。将携带apurine/嘧啶(AP)位点修饰L1 (ALP- au NPs-L1)探针的碱性磷酸酶(ALP)偶联金纳米颗粒固定在异质结表面后,催化发夹组装(CHA)生成的3D双足DNA行走器在miRNA-320d存在下行走,与L1杂交形成双相结构。然后,APE1酶选择性地切割这些双链,触发从电极表面释放ALP-Au NPs。这种空间分离使ALP的催化能力失效,引起明显的光电流衰减。通过协同Cs3Bi2Br9 QDs/BiOBr异质结的特殊PEC性能,APE1酶的特异性识别和高效切割,以及3D步行介导的信号放大,该平台实现了超灵敏的miRNA-320d检测,检测限为0.1 fM,线性范围为1 fM至1 nM。本研究为在PEC生物传感平台中实现新兴钙钛矿材料靶向microRNA检测建立了新的概念框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


