Metal–Oxygen Bonding-Induced Structural Transition Regulation in Co-THQ for High-Performance OER
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Metal–organic frameworks (MOFs) hold significant promise as electrocatalysts for the oxygen evolution reaction (OER) owing to their tunable structure and abundant active sites. However, extensive structural transformation under operating conditions often leads to structural degradation and limited durability. Here, we report a metal–oxygen bonding-induced structural transition regulation by anchoring atomically dispersed ruthenium (Ru) onto 2D conductive MOFs (Ru–Co-THQ). X-ray absorption fine structure and PCOHP revealed that Ru incorporation modulated the local coordination and bonding environment, leading to strengthened Co/Ru–O interactions that can stabilize the MOF framework. In situ Raman and FT-IR spectroscopy revealed that this strengthened Co/Ru–O bonding retarded the reconstruction process, lowered the reconstruction potential, and facilitated the formation of high-valent Co species. As a result, Ru–Co-THQ achieved a low overpotential of 261 mV at 10 mA cm–2 in 1 M KOH, with durability exceeding 300 h and accelerated OER kinetics, outperforming IrO2 and most cobalt-based catalysts. Theoretical calculations revealed strong orbital coupling among Co 3d, Ru 4d, and O 2p orbitals in the reconstructed phases, which modulated the electronic structure, optimized intermediate adsorption, and accelerated kinetics. This work highlights the role of metal–oxygen bonding in regulating structural transition to enhance the MOF-based electrocatalyst activity and durability, offering insights into rational design.
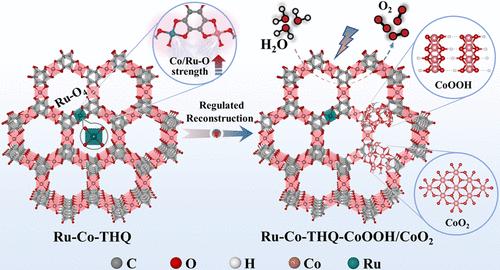
高性能OER中Co-THQ金属-氧键诱导的结构转变调控
金属有机骨架(MOFs)由于其可调的结构和丰富的活性位点,在析氧反应(OER)中具有重要的电催化剂应用前景。然而,在使用条件下,广泛的结构改造往往导致结构退化和有限的耐久性。在这里,我们报道了通过将原子分散的钌(Ru)锚定在二维导电mof (Ru - co - thq)上,金属-氧键诱导的结构转变调控。x射线吸收精细结构和PCOHP表明,Ru的掺入调节了局部配位和成键环境,导致Co/Ru - o相互作用增强,从而稳定了MOF框架。原位拉曼光谱和FT-IR光谱显示,Co/ Ru-O键的增强延缓了重构过程,降低了重构电位,促进了高价Co物质的形成。结果,Ru-Co-THQ在1 M KOH条件下,在10 mA cm-2下实现了261 mV的低过电位,耐久性超过300小时,加速了OER动力学,优于IrO2和大多数钴基催化剂。理论计算表明,重构相中Co 3d、Ru 4d和o2p轨道之间存在强的轨道耦合,从而调节了电子结构,优化了中间吸附,加速了动力学。这项工作强调了金属-氧键在调节结构转变中的作用,以提高基于mof的电催化剂的活性和耐久性,为合理设计提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


