An effective way to produce fatty alcohol through coupling fatty acid with CO2 hydrogenation over CuZnZrOx/C-N catalyst
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The hydrogenation of fatty acids to fatty alcohols, alongside the hydrogenation of CO2 to methanol, represents crucial pathways for addressing the depletion of fossil resources and achieving carbon neutrality. However, the catalytic conversion rates of these two reactions remain low under mild conditions. In this study, we discovered an innovative approach that couples stearic acid (C17COOH) hydrogenation with CO2 hydrogenation in a one-pot catalytic tandem process using the optimized CuZn2ZrOx/C-N catalyst. This method significantly enhances the yields of methanol and fatty alcohol under mild reaction conditions of 200 °C, 3 MPa, 6 h, with a H2:CO2 ratio of 3:1. The reaction mechanism revealed that stearic acid methyl ester (C17COOCH3) acts as a key intermediate in the coupling process. Specifically, the in situ methanol produced from CO2 hydrogenation is converted by C17COOH into C17COOCH3, which is then hydrogenated to form stearic alcohol. This coupling reaction offers important insights for the simultaneous utilization of CO2 and biomass-derived fatty acids in producing value-added chemicals. Furthermore, our findings could inspire the design of more synergistically coupled catalytic reactions that leverage dual-promotional effects for the hydrogenation of CO2 and carboxylic acid compounds. This approach not only enhances the efficiency of these transformations but also aligns with the broader goals of sustainability and resource efficiency in chemical processes.
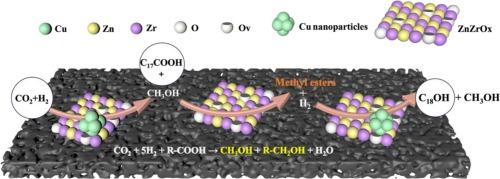
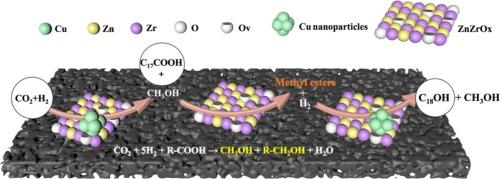
CuZnZrOx/C-N催化剂催化脂肪酸与CO2加氢偶联制备脂肪醇的有效方法
脂肪酸加氢制脂肪醇,以及二氧化碳加氢制甲醇,是解决化石资源枯竭和实现碳中和的关键途径。然而,在温和的条件下,这两种反应的催化转化率仍然很低。在本研究中,我们发现了一种创新的方法,使用优化的CuZn2ZrOx/C-N催化剂,在一锅催化串联过程中将硬脂酸(C17COOH)加氢与CO2加氢偶联。该方法在200 °C、3 MPa、6 h、H2:CO2比为3:1的温和反应条件下,显著提高了甲醇和脂肪醇的收率。反应机理表明硬脂酸甲酯(C17COOCH3)在偶联过程中起关键中间体的作用。具体来说,由CO2加氢产生的原位甲醇被C17COOH转化为C17COOCH3,然后C17COOCH3被加氢生成硬脂醇。这种偶联反应为同时利用二氧化碳和生物质衍生脂肪酸生产增值化学品提供了重要的见解。此外,我们的发现可以启发设计更多协同耦合的催化反应,利用二氧化碳和羧酸化合物加氢的双重促进作用。这种方法不仅提高了这些转换的效率,而且与化学过程中可持续性和资源效率的更广泛目标保持一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


