A Multi-Stage Graph Neural Network–Physics-Informed Neural Network (GNN–PINN) Framework for Thermodynamic Property Prediction
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Accurately predicting thermodynamic properties across various conditions remains a critical challenge, particularly in scenarios involving sparse data or complex molecular interactions. This study proposes a multistage hybrid modeling framework that integrates Graph Neural Networks (GNN) and Physics-Informed Neural Networks (PINN) to predict essential thermodynamic properties, including enthalpy and entropy, for pure substances under various conditions. The model is developed in three distinct stages. First, a GNN encoder captures atomic-level interactions (both bonded and nonbonded) from molecular structures, generating structurally enriched molecular embeddings while leveraging critical constants and reduced state variables through a masking strategy that enables learning from single-phase data sets. Second, a regression submodel utilizes these embeddings to accurately predict saturation pressure (Psat) from molecular structure and temperature, modeling phase equilibrium behavior. Finally, the third stage employs PINN-based fine-tuning, embedding thermodynamic constraints─such as Gibbs free energy equality at phase equilibrium and enthalpy–entropy coupling─as penalties in the loss function to enforce thermodynamic consistency. This integrated GNN–PINN approach accurately predicts vapor- and liquid-phase enthalpies, entropies, and saturation pressures, maintaining robust performance even at equilibrium conditions. The model offers a physically consistent and reliable method for predicting thermodynamic properties, effectively capturing complex molecular interactions while adhering to fundamental physical laws.
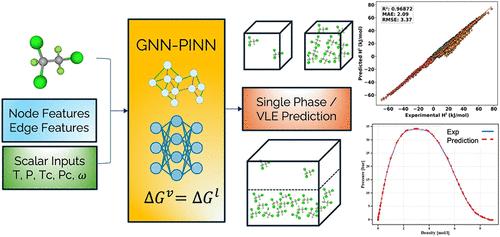
一个多阶段图神经网络-物理信息神经网络(GNN-PINN)框架的热力学性质预测
准确预测各种条件下的热力学性质仍然是一个关键的挑战,特别是在涉及稀疏数据或复杂分子相互作用的情况下。本研究提出了一个多阶段混合建模框架,该框架集成了图神经网络(GNN)和物理信息神经网络(PINN),以预测各种条件下纯物质的基本热力学性质,包括焓和熵。模型的发展分为三个不同的阶段。首先,GNN编码器从分子结构中捕获原子级相互作用(键合和非键合),生成结构丰富的分子嵌入,同时通过屏蔽策略利用关键常数和减少的状态变量,从而能够从单相数据集中学习。其次,回归子模型利用这些嵌入来准确预测分子结构和温度的饱和压力(Psat),模拟相平衡行为。最后,第三阶段采用基于pup的微调,在损失函数中嵌入热力学约束──如相平衡时的吉布斯自由能等式和焓熵耦合──作为惩罚,以加强热力学一致性。这种集成的GNN-PINN方法准确地预测了蒸汽和液相的焓、熵和饱和压力,即使在平衡条件下也能保持稳定的性能。该模型提供了一种物理上一致和可靠的方法来预测热力学性质,有效地捕捉复杂的分子相互作用,同时坚持基本的物理定律。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


