Lithium Nitride-rich Li Protection Layer via Facile Lewis Acid–Base Reaction for Li Metal Batteries
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In this study, we present a scalable approach for forming a lithium nitride (Li3N)-rich protection layer on Li metal, enabling practical applications for high-energy-density lithium metal (Li) batteries. This protection layer is developed through a simple Lewis acid–base reaction involving the direct contact of Li metal with xylylenediamine. This method employs a two-step solution coating process. First, the native oxide layer on the Li surface is removed via chemical polishing. Then, the Li3N protection layer is coated onto the polished Li metal (Li3N@Li). This ensures an effective chemical reaction between Li metal and xylylenediamine, leading to a robust protection layer. The Li nucleation and growth behavior of Li3N@Li shows a dendrite-free morphology with dense and compact properties. Electrochemical evaluations in Li||Li symmetric cells demonstrate dendrite-free Li deposition at 0.5 mA cm–2 for a capacity of 2 mA h cm–2. Additionally, pairing the protected Li anode with a LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode achieves an areal capacity of 5 mA h cm–2. The resulting Li||NCM full cells exhibit stable cycling stability with 78.5% capacity retention and an average Coulombic efficiency of 99.8% for over 100 cycles. This work provides insights into the practical realization of high-energy-density Li metal batteries, offering a scalable solution for developing protection layers on Li metal anodes.
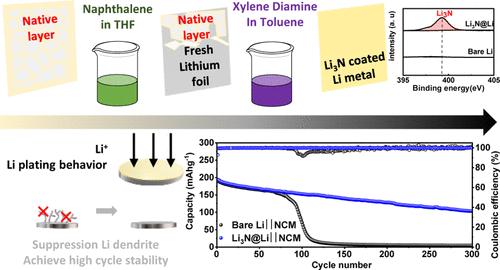
易易路易斯酸碱反应制备富氮化锂保护层的研究
在这项研究中,我们提出了一种可扩展的方法,用于在锂金属上形成富含氮化锂(Li3N)的保护层,从而实现高能量密度锂金属(Li)电池的实际应用。这种保护层是通过一个简单的刘易斯酸碱反应形成的,该反应涉及锂金属与二胺的直接接触。该方法采用两步溶液涂布工艺。首先,通过化学抛光去除锂表面的天然氧化层。然后,将Li3N保护层涂在抛光的Li金属上(Li3N@Li)。这确保了锂金属和二甲二胺之间的有效化学反应,从而形成坚固的保护层。Li3N@Li的Li形核和生长行为表现为无枝晶形态,具有致密致密的性质。在Li||锂对称电池中的电化学评价表明,在0.5 mA cm-2下,无枝晶的锂沉积容量为2 mA h cm-2。此外,将受保护的锂阳极与LiNi0.8Co0.1Mn0.1O2 (NCM811)阴极配对可获得5 mA h cm-2的面容量。得到的Li||NCM全电池具有稳定的循环稳定性,容量保持率为78.5%,平均库仑效率为99.8%,循环次数超过100次。这项工作为高能量密度锂金属电池的实际实现提供了见解,为在锂金属阳极上开发保护层提供了可扩展的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


