Revealing the spin-polarization-induced d-band splitting effect of Fe-series atoms on the dehydrogenation performance of Pt/TiO2 catalyst for Dodecahydro-N-ethylcarbazole
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Liquid organic hydrogen carriers (LOHC) technology has emerged as one of the most promising novel hydrogen storage approaches, whose bottleneck is the need to develop efficient and low-cost dehydrogenation catalysts. Among various catalytic systems, Pt-based bimetallic catalysts have attracted significant research attention due to their superior catalytic performance and potential for cost reduction. In this study, we conducted a systematic investigation into the influence of the introduction of Fe-series atoms (Fe, Co, Ni) on 12H-NECZ dehydrogenation reactions of Pt/TiO2 catalyst. The results demonstrate that the introduced Fe-series atoms facilitate enhanced orbital hybridization, particularly between their 3d orbitals and the Pt-5d orbitals, leading to a pronounced spin polarization effect, modulating the d-band splitting (Δεd). These atoms have excellent electron migration properties, which increase the electron density around the Pt atoms. This electronic restructuring modulates the dual d-band centers of the metal Pt, weakens the strong adsorption of reaction intermediates/products and dramatically reduces the energy barrier of the breaking of C–H bonds. Specifically, the rate-determining-step (RDS) barrier of the most effective Pt3Co/TiO2 catalyst is 0.43 eV lower than that of Pt/TiO2, which is attributed to the moderate spin polarization strength and optimal electron structure, balancing the adsorption of intermediates/products (εd↑) and hydrogen (εd↓). This study establishes a theoretical foundation for the design of cost-effective, high-performance dehydrogenation catalysts for LOHC.
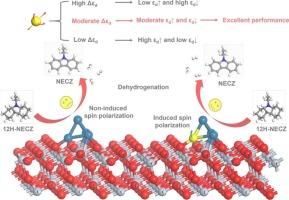
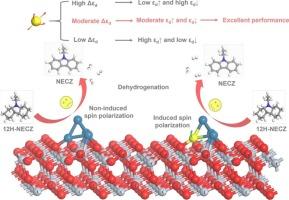
揭示了fe系原子自旋极化诱导的d带分裂对十二氢- n-乙基咔唑Pt/TiO2催化剂脱氢性能的影响
液态有机氢载体(LOHC)技术是目前最具发展前景的新型储氢技术之一,其瓶颈在于开发高效、低成本的脱氢催化剂。在各种催化体系中,pt基双金属催化剂因其优越的催化性能和降低成本的潜力而备受关注。本研究系统研究了Fe系原子(Fe, Co, Ni)的引入对Pt/TiO2催化剂12H-NECZ脱氢反应的影响。结果表明,fe系原子的引入促进了轨道杂化的增强,特别是在它们的3d轨道和Pt-5d轨道之间,导致了明显的自旋极化效应,调节了d带分裂(Δεd)。这些原子具有优异的电子迁移特性,这增加了Pt原子周围的电子密度。这种电子重组调节了金属Pt的双d带中心,减弱了反应中间体/产物的强吸附,显著降低了C-H键断裂的能垒。特别的是,最有效的Pt3Co/TiO2催化剂的RDS势阱比Pt/TiO2低0.43 eV,这是由于其自旋极化强度适中,电子结构最优,平衡了中间产物(εd↑)和氢(εd↓)的吸附。本研究为设计高性价比、高性能的LOHC脱氢催化剂奠定了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


