Thermosensitive chitosan-oxidized dextran-β-glycerophosphate sodium injectable hydrogel for enhanced solubility and prolonged release of ethinylestradiol: a novel long-acting contraceptive platform
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-22
DOI:10.1016/j.ejpb.2025.114872
引用次数: 0
Abstract
Ethinylestradiol (EE) is a clinically used estrogen with antifertility effects that is poorly water soluble, limiting bioavailability and preventing long-term release when administered orally. This study addresses these challenges through the development of a novel injectable thermosensitive hydrogel that both improves hydrophobic EE dispersion and enables sustained in vivo release, presenting significant potential for long-term contraception. Carboxymethyl-β-cyclodextrin (CM-β-CD) was synthesized to encapsulate EE, thereby enhancing its solubility and distribution in the hydrogel matrix. We found that hydrogels prepared by adding 0.5% (w/v) oxidized dextran (ODex) to the chitosan/sodium β-glycerophosphate (CS/ODex/β-GP) system exhibited mechanical strength favorable for cell growth compared to conventional CS/β-GP, resolved the problems of poor stability and mechanical strength of CS/β-GP hydrogels, and ensured effective retention and delivery in vivo. A 240h drug release study demonstrated the hydrogel’s ability to sustain release, highlighting its potential to prolong contraceptive efficacy. Biocompatibility testing showed cell viability in excess of 85%. FTIR and SEM characterization further confirmed the functional structure and morphology of the hydrogel. This thermosensitive injection system provides a promising platform for the long-term release of hydrophobic drugs such as EE, providing an innovative approach to extended contraceptive delivery.
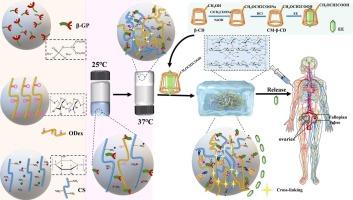
热敏壳聚糖氧化葡聚糖-β-甘油磷酸钠注射水凝胶增强溶解度和延长释放乙炔雌二醇:一个新的长效避孕平台。
炔雌醇(EE)是临床上使用的雌激素,具有抗生育作用,水溶性差,限制生物利用度,口服时不能长期释放。本研究通过开发一种新型可注射热敏水凝胶来解决这些挑战,这种水凝胶既能改善疏水EE的分散,又能在体内持续释放,具有长期避孕的巨大潜力。合成羧甲基-β-环糊精(CM-β-CD)包封EE,提高其在水凝胶基质中的溶解度和分布。结果表明,在壳聚糖/β-甘油磷酸钠(CS/ODex/β-GP)体系中添加0.5% (w/v)氧化右旋糖酐(ODex)制备的水凝胶与常规CS/β-GP相比,具有有利于细胞生长的机械强度,解决了CS/β-GP水凝胶稳定性差、机械强度差的问题,并保证了水凝胶在体内的有效保留和递送。一项240小时的药物释放研究表明,水凝胶具有持续释放的能力,突出了其延长避孕效果的潜力。生物相容性试验表明,细胞存活率在85%以上。FTIR和SEM表征进一步证实了水凝胶的功能结构和形态。这种热敏注射系统为疏水药物(如EE)的长期释放提供了一个有前景的平台,为延长避孕药的递送提供了一种创新的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


