Rapid molecular diagnostic method for Gardnerella vaginalis based on CRISPR-Cas12a and recombinase-aided amplification (RAA)
IF 2.9
3区 医学
Q2 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
Abstract
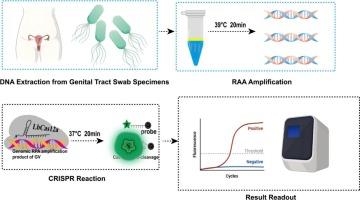
Imbalance of the vaginal microbiota, particularly the overgrowth of Gardnerella vaginalis, is the primary cause of bacterial vaginosis (BV), which poses a significant threat to women's reproductive health. Therefore, early and rapid diagnosis of BV is crucial. Current laboratory diagnostic methods for BV mainly rely on Amsel's clinical criteria, bacterial culture, and PCR techniques. However, these methods have notable limitations: Amsel's criteria are subject to operator subjectivity, culture methods are time-consuming and require specialized expertise, while PCR necessitates expensive instrumentation. These constraints hinder their widespread clinical application. To address this issue, developing a highly accurate and low-cost molecular diagnostic method holds significant clinical value for BV detection. In recent years, recombinase-aided amplification (RAA) and CRISPR-Cas12a gene-editing technologies have achieved groundbreaking progress in nucleic acid detection. This study innovatively integrates RAA isothermal amplification with CRISPR-Cas12a detection to successfully establish a rapid nucleic acid detection platform for Gardnerella vaginalis. Experimental results demonstrate that this platform achieves a detection sensitivity of 10 copies/mL for Gardnerella vaginalis genomic DNA, with no cross-reactivity against other common reproductive tract pathogens. In validation tests using 44 clinical vaginal swab samples, the platform showed a 100.00 % positive agreement rate compared to qPCR. These findings confirm that the CRISPR-Cas12a-based detection platform exhibits excellent specificity, sensitivity, and reliability, serving as an effective tool for monitoring Gardnerella vaginalis colonization levels. This approach provides a novel molecular diagnostic solution for early BV screening and prevention.
基于CRISPR-Cas12a和重组酶辅助扩增(RAA)的阴道加德纳菌快速分子诊断方法
阴道微生物群失衡,特别是阴道加德纳菌的过度生长,是细菌性阴道病的主要原因,对妇女的生殖健康构成重大威胁。因此,早期和快速诊断细菌性阴道炎至关重要。目前BV的实验室诊断方法主要依靠Amsel临床标准、细菌培养和PCR技术。然而,这些方法有明显的局限性:Amsel的标准受制于操作者的主观性,培养方法耗时且需要专业知识,而PCR需要昂贵的仪器。这些限制阻碍了其广泛的临床应用。为解决这一问题,开发一种高精度、低成本的分子诊断方法对细菌性阴道炎的检测具有重要的临床价值。近年来,重组酶辅助扩增(RAA)和CRISPR-Cas12a基因编辑技术在核酸检测方面取得了突破性进展。本研究创新性地将RAA等温扩增与CRISPR-Cas12a检测相结合,成功建立了阴道加德纳菌的快速核酸检测平台。实验结果表明,该平台对阴道加德纳菌基因组DNA的检测灵敏度为10拷贝/mL,对其他生殖道常见病原体无交叉反应性。在使用44个临床阴道拭子样本的验证测试中,与qPCR相比,该平台显示100.00 %的阳性符合率。这些发现证实,基于crispr - cas12的检测平台具有出色的特异性、敏感性和可靠性,可作为监测阴道加德纳菌定殖水平的有效工具。该方法为细菌性阴道炎的早期筛查和预防提供了新的分子诊断方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Clinica Chimica Acta
医学-医学实验技术
CiteScore
10.10
自引率
2.00%
发文量
1268
审稿时长
23 days
期刊介绍:
The Official Journal of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Clinica Chimica Acta is a high-quality journal which publishes original Research Communications in the field of clinical chemistry and laboratory medicine, defined as the diagnostic application of chemistry, biochemistry, immunochemistry, biochemical aspects of hematology, toxicology, and molecular biology to the study of human disease in body fluids and cells.
The objective of the journal is to publish novel information leading to a better understanding of biological mechanisms of human diseases, their prevention, diagnosis, and patient management. Reports of an applied clinical character are also welcome. Papers concerned with normal metabolic processes or with constituents of normal cells or body fluids, such as reports of experimental or clinical studies in animals, are only considered when they are clearly and directly relevant to human disease. Evaluation of commercial products have a low priority for publication, unless they are novel or represent a technological breakthrough. Studies dealing with effects of drugs and natural products and studies dealing with the redox status in various diseases are not within the journal''s scope. Development and evaluation of novel analytical methodologies where applicable to diagnostic clinical chemistry and laboratory medicine, including point-of-care testing, and topics on laboratory management and informatics will also be considered. Studies focused on emerging diagnostic technologies and (big) data analysis procedures including digitalization, mobile Health, and artificial Intelligence applied to Laboratory Medicine are also of interest.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


