Incorporating maternal metabolome and genome to understand the effects of prenatal phenol exposure on the first 1000-day growth in an exposome-based study
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Previous studies on prenatal environmental factors and early-life growth generally applied the single-exposure strategy, mostly relying on cross-sectional growth indicators. No attempt has been made to explore the environmental effects on longitudinal growth at the exposome level. Further, the underlying bio-mechanisms and gene-environment interactions are poorly understood. Within 1,944 mother–child pairs from the Shanghai Birth Cohort, neonatal sex-specific z-scores of weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length (WLZ) were used as the fetal growth outcomes. The growth trajectories of WAZ, LAZ, and WLZ before 2 years old were used as the child growth outcomes. We combined multiple exposome analysis strategies to screen for prenatal environmental factors that continuously affect the first 1000-day growth, based on which maternal metabolome and genome were incorporated to explore the underlying bio-mechanisms and effect modification by genetic predisposition. We found that phenols were the environmental factors that had a lasting growth impact, showing negative associations with neonatal WAZ, LAZ, and WLZ and positive associations with the risks of slow WAZ and LAZ growth trajectories and rapid WLZ growth trajectory in children. Metabolomic and gene-environment interaction analyses suggested the chemicals may exert such lasting effects by upregulating maternal glucose and lipid metabolism during pregnancy, and the effects were specifically more pronounced in mothers with high glucose and low lipid genetic predispositions. Our study suggests that prenatal exposure to phenols has lasting effects on offspring’s first-1000-day growth by disturbing maternal glucose and lipid metabolism. The effects may be aggravated by maternal high glucose and low lipid genetic predispositions.
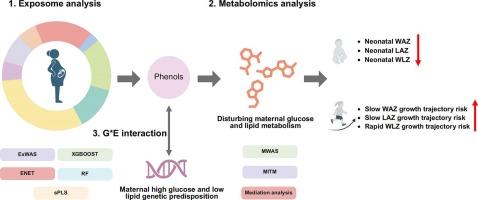
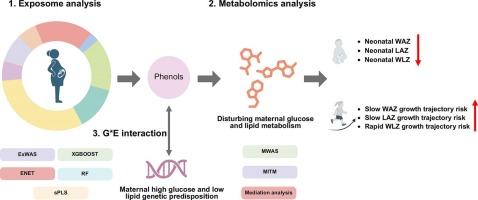
在一项基于暴露体的研究中,结合母体代谢组和基因组来了解产前苯酚暴露对头1000天生长的影响
以往关于产前环境因素与生命早期生长的研究一般采用单一暴露策略,多依赖于横断面生长指标。没有人试图在暴露水平上探讨环境对纵向生长的影响。此外,潜在的生物机制和基因与环境的相互作用尚不清楚。在来自上海出生队列的1944对母婴中,新生儿性别特异性的年龄体重(WAZ)、年龄长度(LAZ)和体重长度(WLZ) z分数被用作胎儿生长结局。以WAZ、LAZ和WLZ在2 岁前的生长轨迹作为儿童生长结局。我们结合多种暴露分析策略,筛选持续影响前1000天生长的产前环境因素,并在此基础上结合母体代谢组和基因组,探索潜在的生物机制和遗传易感的影响改变。我们发现,酚类物质是对生长具有持久影响的环境因素,与新生儿WAZ、LAZ和WLZ呈负相关,与儿童WAZ和LAZ缓慢生长轨迹和WLZ快速生长轨迹的风险呈正相关。代谢组学和基因-环境相互作用分析表明,这些化学物质可能通过上调妊娠期间母亲的葡萄糖和脂质代谢而产生这种持久的影响,并且这种影响在高糖和低脂遗传易感性的母亲中更为明显。我们的研究表明,产前暴露于酚类物质通过扰乱母体葡萄糖和脂质代谢,对后代前1000天的生长有持久的影响。这种影响可能会因母亲的高血糖和低脂遗传倾向而加重。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


