Catenibacterium mitsuokai promotes hepatocellular carcinogenesis by binding to hepatocytes and generating quinolinic acid
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The role of gut microbes in the pathogenesis of hepatocellular carcinoma (HCC) remains unclear. Here, we identified that Catenibacterium is enriched in both the feces and tumors of patients with HCC. C. mitsuokai accelerated HCC carcinogenesis in both conventional and germ-free mice. Furthermore, C. mitsuokai disrupted the gut barrier and translocated to the liver as live bacteria. Critically, the C. mitsuokai surface protein Gtr1/RagA interacts with the γ-catenin receptor on HCC cells, facilitating its attachment and colonization in the mouse liver. We further revealed that the pro-tumorigenic effect of C. mitsuokai depends on its secreted metabolite, quinolinic acid. Mechanistically, quinolinic acid binds to and activates the tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2 (TIE2) on HCC cells. Phosphorylated TIE2 subsequently activates the downstream oncogenic phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway, thereby promoting HCC progression. In summary, C. mitsuokai disrupts the gut barrier, colonizes HCC cells via Gtr1/RagA-γ-catenin, and secretes quinolinic acid, which binds to TIE2 and drives the PI3K/AKT pathway to promote HCC development.
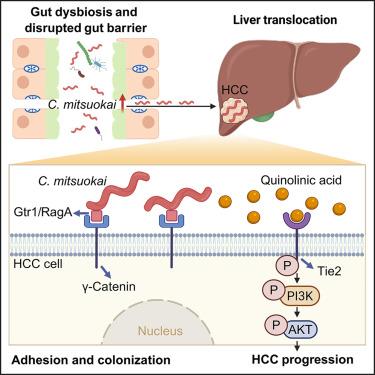
Catenibacterium mitsuokai通过与肝细胞结合并产生喹啉酸促进肝细胞癌变
肠道微生物在肝细胞癌(HCC)发病机制中的作用尚不清楚。在这里,我们发现Catenibacterium在HCC患者的粪便和肿瘤中都富集。C. mitsuokai在常规小鼠和无菌小鼠中加速HCC癌变。此外,C. mitsuokai破坏了肠道屏障,并作为活细菌转移到肝脏。关键是,C. mitsuokai表面蛋白Gtr1/RagA与HCC细胞上的γ-连环蛋白受体相互作用,促进其在小鼠肝脏中的附着和定植。我们进一步发现,光脉草的促肿瘤作用取决于其分泌的代谢物喹啉酸。在机制上,喹啉酸结合并激活肝癌细胞上具有免疫球蛋白和表皮生长因子同源结构域2 (TIE2)的酪氨酸激酶。磷酸化的TIE2随后激活下游致癌磷脂酰肌醇3-激酶/蛋白激酶B (PI3K/AKT)通路,从而促进HCC进展。综上所述,C. mitsuokai破坏肠道屏障,通过Gtr1/RagA-γ-catenin定植HCC细胞,分泌喹啉酸,与TIE2结合,驱动PI3K/AKT通路,促进HCC的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


