Fast Nucleophilic Approach to Exhaustively Substituted Porphyrin Derivatives at the “Eastern Half”
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
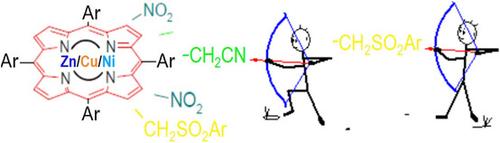
meso-Tetraarylporphyrin chelates (of Zn2+, Ni2+, and Cu2+) are easily available from parent porphyrins. Their reactions with nitric acid (yellow fuming HNO3, d = 1.52, diluted to 25%–50%), under optimized conditions in CHCl3 at room temperature, can be carried out selectively, thus giving mainly β,β-dinitro-substituted moieties (usually two or three isomers of the above complexes). These intermediates (obtained in overall yields of up to 73%), upon the reaction with carbanions bearing a leaving group X at the reactive center, afford the target products, the ones of vicarious nucleophilic substitution (VNS) of hydrogen. This approach allows the synthesis of porphyrin derivatives exhaustively β-substituted in the so-called “Eastern half”, starting from a simple meso-tetraarylporphyrins. These types of products are sought in anticancer photodynamic therapy (PDT). Thus, the above two-step procedure including very attractive VNS reaction gives hydrophilic or amphiphilic moieties that seem to be potential PDT agents with unique structures, being practically unavailable by any other alternative methods.
“东半部”全取代卟啉衍生物的快速亲核方法
中四芳基卟啉螯合物(Zn2+, Ni2+和Cu2+)很容易从母体卟啉中得到。它们与硝酸(发黄的HNO3, d = 1.52,稀释至25% ~ 50%)在室温CHCl3中,在优化条件下选择性反应,主要生成β、β-二硝基取代基团(通常为上述配合物的两种或三种异构体)。这些中间体(总产率高达73%)与在反应中心带有离去基X的碳原子反应后,产生目标产物,即氢的替代亲核取代(VNS)产物。这种方法允许从简单的中四芳基卟啉开始,在所谓的“东半”中完整地合成β-取代的卟啉衍生物。这些类型的产品在抗癌光动力疗法(PDT)中被寻求。因此,上述两步过程包括非常吸引人的VNS反应,可以得到具有独特结构的亲水或两亲性基团,这些基团似乎是潜在的PDT试剂,实际上是任何其他替代方法都无法获得的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


