Stable proton storage in α-MoO3 via a bifunctional molecular crowding electrolyte
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
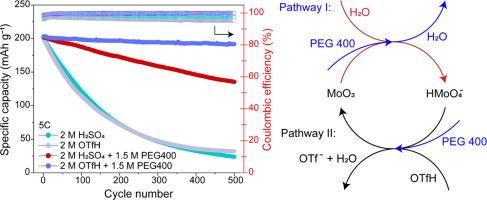
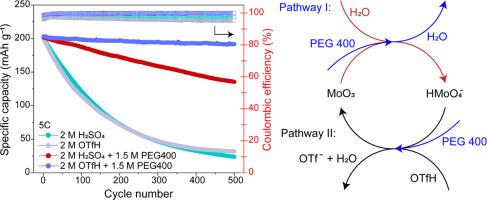
Layered molybdenum trioxide (α-MoO3) storing protons exhibits a high theoretical capacity and extraordinary rate capability and can be used as a reliable anode material for aqueous proton batteries. But a severe dissolution issue during operation always results in rapid failure in battery performance. Herein, a bifunctional molecular crowding electrolyte was engineered to address this issue. The high-concentration inert polymer, polyethylene glycol 400, reduces the free water activity of the electrolyte and the low-concentration solute, trifluoromethanesulfonic acid, forms a proton-rich electrolyte environment under such a water-poor condition thanks to its intrinsic superacidity, both of which favour the reverse shift of the dissolution equilibrium, and with the latter enabling the electrolyte with high ion conductivity as well. Thus, the α-MoO3 electrode in the above electrolyte shows a longer lifespan without sacrificing its rate capability. The analysis on charge storage mechanisms by X-ray powder diffraction technique demonstrates that α-MoO3 undergoes bare proton-involved multistep two-phase and solid-solution reactions, where only the two-phase reaction between hydrogen molybdenum bronze II and III phases and the solid-solution reactions of themselves are reversible.
双功能分子拥挤电解质在α-MoO3中的稳定质子存储
层状三氧化钼(α-MoO3)存储质子具有较高的理论容量和非凡的倍率性能,可作为一种可靠的水性质子电池负极材料。但在使用过程中,严重的溶解问题往往会导致电池性能迅速失效。本文设计了一种双功能分子拥挤电解质来解决这一问题。高浓度的惰性聚合物聚乙二醇400降低了电解质的自由水活度,而低浓度的溶质三氟甲烷磺酸由于其固有的超酸性,在这种贫水条件下形成了富质子的电解质环境,两者都有利于溶解平衡的反向移动,后者也使电解质具有高离子导电性。因此,上述电解质中的α-MoO3电极在不牺牲其倍率能力的情况下表现出更长的使用寿命。x射线粉末衍射技术对电荷储存机理的分析表明,α-MoO3发生了裸质子参与的多步两相固溶反应,其中只有氢钼青铜II、III相的两相反应和自身的固溶反应是可逆的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


