Dual-Single-Atom Ruthenium–Copper Anchored on Magnesium–Aluminum Layered Double Hydroxide Enhancing Dual-Enzymatic Activities for Synergistic Anti-Liver Cancer Therapy
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Single-atom nanozymes (SANs), with their tunable metal active centers, enable the modulation of various enzyme activities for antitumor therapy. However, these materials encounter substantial challenges in cancer therapy owing to their limited biocompatibility and biodegradability. Additionally, the high-temperature pyrolysis process involved in their synthesis significantly restricts their practical, large-scale application. To address these challenges, we propose the construction of an LDH-based SAN exhibiting peroxidase-like (POD-like) activity and glutathione depletion capability. We successfully developed a SAN containing ruthenium (Ru) and copper (Cu) bimetallic atoms (MgAl-LDH/Ru/Cu), where Ru and Cu are distributed in single-atom dispersed states, forming nanodomains. The synergistic effects of the Ru/Cu bimetallic system enable MgAl-LDH/Ru/Cu to demonstrate superior POD-like and glutathione peroxidase-like (GPx-like) catalytic activities compared to those of MgAl-LDH/Ru or MgAl-LDH/Cu alone. Density functional theory calculations indicate that both Ru and Cu sites in MgAl-LDH/Ru/Cu exhibit lower energy barriers for POD-like and GPx-like reactions, likely due to enhanced electron loss states at the Ru/Cu bimetallic single-atom sites compared to those at Ru or Cu sites individually. Both in vitro and in vivo experiments demonstrate that the Ru/Cu bimetallic loading significantly outperforms the individual metal loadings of Ru or Cu in terms of anti-liver cancer efficacy. Importantly, by leveraging the precise correlation between the catalytic structural unit of MgAl-LDH-based SANs and enzyme activity, we further elucidated the potential contributions of POD-like and GPx-like activities to cancer therapy. MgAl-LDH-based multimetal SAN represents a synergistic multienzyme nanoplatform with a tunable elemental composition, paving the way for further investigations into the interplay between multienzyme activity and antitumor effects.
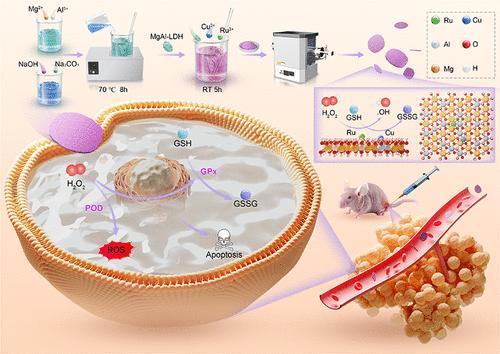
双单原子钌铜锚定在镁铝层状双氢氧化物上增强双酶活性协同抗肝癌治疗
单原子纳米酶(SANs)具有可调节的金属活性中心,能够调节各种酶的活性,用于抗肿瘤治疗。然而,由于其有限的生物相容性和生物可降解性,这些材料在癌症治疗中遇到了实质性的挑战。此外,合成过程中的高温热解过程极大地限制了它们的实际大规模应用。为了解决这些挑战,我们提出了一种基于ldh的SAN,具有过氧化物酶样(pod样)活性和谷胱甘肽消耗能力。我们成功地开发了一种含有钌(Ru)和铜(Cu)双金属原子(MgAl-LDH/Ru/Cu)的SAN,其中Ru和Cu以单原子分散状态分布,形成纳米畴。与单独的MgAl-LDH/Ru或MgAl-LDH/Cu相比,Ru/Cu双金属体系的协同效应使MgAl-LDH/Ru/Cu表现出更好的pod样和谷胱甘肽过氧化物酶样(gpx样)的催化活性。密度泛函理论计算表明,MgAl-LDH/Ru/Cu中的Ru和Cu位在类pod和类gx反应中表现出较低的能垒,这可能是由于Ru/Cu双金属单原子位上的电子损失态比Ru或Cu位上的电子损失态增强。体外和体内实验均表明,Ru/Cu双金属负载在抗肝癌功效方面明显优于单个金属负载Ru或Cu。重要的是,通过利用mgal - ldh基SANs的催化结构单元与酶活性之间的精确相关性,我们进一步阐明了pod样和gpx样活性对癌症治疗的潜在贡献。基于mgal - ldh的多金属SAN代表了一种具有可调元素组成的协同多酶纳米平台,为进一步研究多酶活性与抗肿瘤作用之间的相互作用铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


