Tuning Li/Al-LDHs nucleation and active sites toward enhanced lithium extraction
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Lithium–aluminum layered double hydroxides (Li/Al-LDHs) have achieved successful industrial-scale application in lithium extraction from salt lakes due to their excellent structural stability, high selectivity and environmental friendliness. However, they suffer from limited adsorption capacity. Herein, this issue can be effectively resolved by precise regulation of the LDH nucleation strategy, thereby controlling oxygen species content. The Li/Al-LDHs are synthesized via a one-pot precipitation method, and the nucleation conditions, including Al/Li ratio, crystallization temperature and terminal pH, are systematically optimized through crystal growth analysis, adsorption performance evaluation, and structural stability characterization. The results confirm that the adsorption capacity of Li/Al-LDHs is critically dependent on the pH value attained at equilibrium. Notably, the terminal pH simultaneously governs Al(OH)3 nucleation and Li+ intercalation through oxygen speciation control. The elevated active oxygen content (38.65%) enhances hydrophilicity (contact angle 22.5°) and strengthens lithium-ion diffusion kinetics. The density functional theory (DFT) calculation further indicates that high levels of active oxygen (37.5%) facilitate low formation energy (−3.527 eV) and adsorption energies (−0.739 eV), confirming the correlation between the structure and Li active sites. What's more, the prepared Li/Al-LDHs show outstanding lithium adsorption performance with a maximum adsorption capacity of 13.42 mg g−1 in East Taijinar salt-lake brine. The separation coefficients of Na+, K+, and Mg2+ are 395.86, 140.89, and 121.69, respectively. This study provides vital insights for advancing high-performance aluminum-based lithium adsorbents.
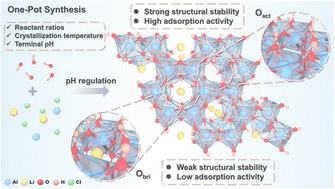
调整Li/Al-LDHs成核和活性位以增强锂萃取
锂铝层状双氢氧化物(Li/Al-LDHs)由于其优异的结构稳定性、高选择性和环境友好性,在盐湖提锂中获得了成功的工业规模应用。然而,它们的吸附能力有限。在此,通过精确调控LDH成核策略,从而控制氧的含量,可以有效地解决这一问题。采用一锅沉淀法合成Li/Al- ldhs,并通过晶体生长分析、吸附性能评价和结构稳定性表征,系统优化了Al/Li比、结晶温度和末端pH等成核条件。结果证实Li/Al-LDHs的吸附能力与平衡时的pH值密切相关。值得注意的是,末端pH值通过氧形态控制同时控制Al(OH)3成核和Li+嵌入。活性氧含量升高(38.65%)增强了亲水性(接触角22.5°),增强了锂离子扩散动力学。密度泛函理论(DFT)计算进一步表明,高水平的活性氧(37.5%)有利于较低的地层能(- 3.527 eV)和吸附能(- 0.739 eV),证实了结构与Li活性位点之间的相关性。制备的Li/Al-LDHs在台湾东部盐湖卤水中表现出优异的锂吸附性能,最大吸附量为13.42 mg g−1。Na+、K+和Mg2+的分离系数分别为395.86、140.89和121.69。这项研究为推进高性能铝基锂吸附剂提供了重要的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


