A Nitroreductase-Activated Chemiluminescent Prodrug for Real-Time Monitoring of Camptothecin Release in Peritoneal Metastasis Theranostics
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
While most theranostic prodrugs utilize fluorescence for reporting, chemiluminescence offers superior signal-to-noise ratios. However, chemiluminescence-based prodrug systems remain relatively scarce. Herein, we report the development of CPT-NBz-CL, a novel hypoxia-responsive theranostic prodrug activated by nitroreductase (NTR) via a self-immolative linker mechanism. This activation concomitantly releases a potent chemotherapeutic camptothecin (CPT) and generates a robust chemiluminescence signal for reporting. Encapsulation of CPT-NBz-CL into nanoparticles (CPT-NBz-CL NPs) facilitated in vivo application. In vitro studies demonstrated hypoxia-selective cytotoxicity and chemiluminescence generation. In a murine 4T1-Luc1 peritoneal metastasis model, CPT-NBz-CL NPs delineated hypoxic tumor regions through in vivo chemiluminescence imaging, reflecting the endogenous NTR activity. Crucially, CPT-NBz-CL NPs showed antitumor efficacy comparable to free CPT while significantly mitigating the parent drug’s systemic toxicity. This work validates an NTR-triggered, self-immolative chemiluminescence platform as a promising strategy for integrating high-sensitivity imaging with potent, targeted cancer therapy, offering an improved safety profile.
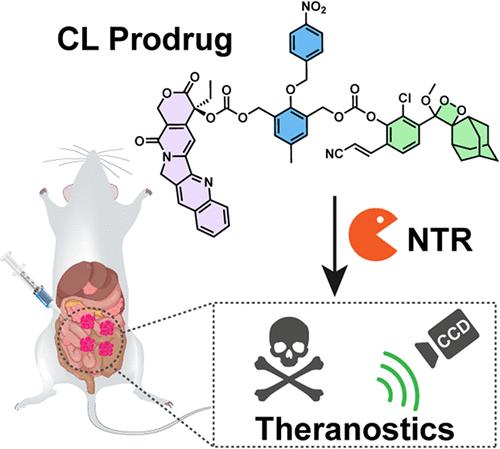
一种硝基还原酶激活的化学发光前药用于腹膜转移治疗中喜树碱释放的实时监测
虽然大多数治疗前药使用荧光报告,化学发光提供优越的信噪比。然而,基于化学发光的前药系统仍然相对稀缺。在此,我们报道了CPT-NBz-CL的开发,这是一种新型的缺氧反应性治疗前药,由硝基还原酶(NTR)通过自我牺牲的连接机制激活。这种激活同时释放出一种有效的化疗喜树碱(CPT),并产生一个强大的化学发光信号。将CPT-NBz-CL包封成纳米颗粒(CPT-NBz-CL NPs)有利于体内应用。体外研究证实了低氧选择性细胞毒性和化学发光的产生。在小鼠4T1-Luc1腹膜转移模型中,CPT-NBz-CL NPs通过体内化学发光成像描绘了低氧肿瘤区域,反映了内源性NTR活性。关键是,CPT- nbz - cl NPs显示出与游离CPT相当的抗肿瘤功效,同时显著减轻母体药物的全身毒性。这项工作验证了ntr触发的自焚化学发光平台作为一种有前途的策略,将高灵敏度成像与有效的靶向癌症治疗相结合,提供了更好的安全性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


