Discovery of Potent and Selective Reversible Ubiquitin-Like Modifier Activating Enzyme 5 Inhibitors Targeting the UFMylation Pathway
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
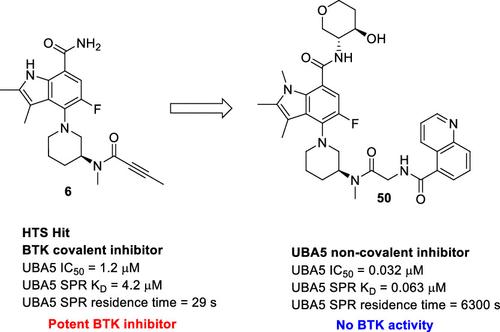
Ubiquitin-like modifier activating enzyme 5 (UBA5), an E1 enzyme in the UFMylation pathway, is of interest as a therapeutic target in diseases such as cancer due to the critical role that UFMylation plays in cellular function. Strategic structure–activity relationship optimization of high-throughput screen hit 6, a Bruton’s tyrosine kinase covalent inhibitor, was conducted by using both conventional and microscale library synthesis. This approach identified 49 and 50 as potent and selective noncovalent UBA5 inhibitors with significantly improved surface plasmon resonance and nanodifferential scanning fluorimetry biophysical profiles. Cellular target engagement for 49 and 50 was confirmed by a cellular thermal shift assay and translated to inhibition of UFMylation of UBA5 and UFC1 in retinal pigment epithelial-1 cells as well as reduction of UFMylation of the downstream protein UFMylation substrate RPL26. Furthermore, 49 and 50 demonstrated specificity for UBA5 among the other E1–E2 transesterification pathways. In this communication, we report the discovery and synthesis of potent and selective UBA5 inhibitors, providing valuable tool compounds for studying the UFMylation pathway.
靶向ufmy化途径的有效和选择性可逆泛素样修饰剂激活酶5抑制剂的发现
泛素样修饰物激活酶5 (UBA5)是ufmyation通路中的一种E1酶,由于ufmyation在细胞功能中起着关键作用,它被认为是癌症等疾病的治疗靶点。采用常规文库合成和微尺度文库合成两种方法对布鲁顿酪氨酸激酶共价抑制剂screen hit 6进行了战略性构效关系优化。该方法确定49和50是有效的选择性非共价UBA5抑制剂,具有显著改善的表面等离子体共振和纳米差示扫描荧光学生物物理谱。细胞热移实验证实了49和50的细胞靶标作用,并解释为抑制视网膜色素上皮-1细胞中UBA5和UFC1的ufmyation,以及下游蛋白ufmyation底物RPL26的ufmyation。此外,49和50在其他E1-E2酯交换途径中表现出对UBA5的特异性。在这篇通讯中,我们报道了强效和选择性UBA5抑制剂的发现和合成,为研究ufmyation途径提供了有价值的工具化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


