Ir-enabled reductive hydroamination of alkynes with nitroarenes
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Direct catalytic reductive hydroamination of alkynes enables C-N bond formation. In comparison with traditional reductive hydroamination of alkynes with amines, using nitroarenes as amine sources remains underdeveloped. Herein, we report a new and practical Ir-catalyzed muti-step tandem reductive hydroamination of alkynes with nitroarenes, delivering a series of aromatic amines in good yields (up to 96 %) under mild conditions. In addition, the alkynes can be incorporated into nitro-substituted bioactive molecules and are readily realized structural modification.

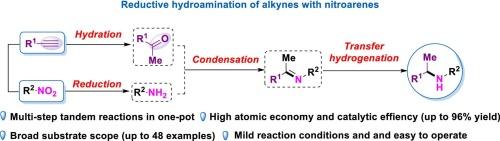
炔与硝基芳烃的红外还原氢胺化反应
炔的直接催化还原氢胺化反应使C-N键形成。与传统的炔与胺的还原氢胺化反应相比,硝基芳烃作为胺源的研究还不发达。在此,我们报道了一种新的实用的ir催化炔与硝基芳烃的多步串联还原氢胺化反应,在温和的条件下以高收率(高达96%)产生一系列芳香胺。此外,炔烃可以掺入到硝基取代的生物活性分子中,并且易于实现结构修饰。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


