A dual-channel self-amplifying lateral flow immunoassay for the on-site ultrasensitive simultaneous detection of salivary cortisol and melatonin
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The on-site simultaneous measurement of cortisol and melatonin is valuable for comprehensively assessing circadian rhythms and understanding their complex interplay under physiological conditions. However, no commercial point-of-care test currently enables the concurrent detection of these salivary hormones. Consequently, there is a significant demand for an on-site, user-friendly diagnostic platform. While the lateral flow immunoassay (LFI) is widely used in point-of-care testing, its sensitivity is insufficient for detecting salivary cortisol and melatonin. To overcome this limitation, the self-amplifying LFI (saLFI) developed previously for salivary cortisol detection, is extended to enable salivary melatonin detection in combination with a gold enhancement method. Two saLFIs are integrated into a single device using a dual-channel sample pad, creating a dual-channel self-amplifying lateral flow immunoassay (2ch-saLFI). This integrated dual-channel sample pad provides an equal distribution of the sample solution to two strips and two self-amplifying complexes. Consequently, the novel 2ch-saLFI enables the simultaneous on-site detection of both hormones from a single sample injection, delivering results within 30 min. The 2ch-saLFI demonstrates a high sensitivity, with detection limits of 5.39 and 0.476 pg mL−1 for cortisol and melatonin, respectively (i.e., below typical physiological levels). Validation using 20 clinical samples provides a strong correlation with standard ELISA methods (R2 = 0.9017 for cortisol and 0.9101 for melatonin). Overall, the 2ch-saLFI represents the first point-of-care test enabling the on-site, simultaneous, and highly sensitive measurement of salivary cortisol and melatonin via a single sample injection. This development allows an affordable clinical assessment of circadian biology for advanced healthcare applications.

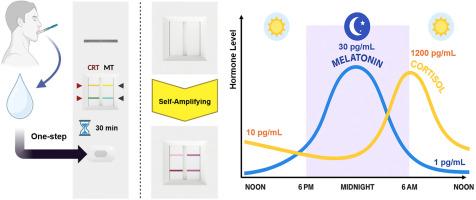
双通道自放大侧流免疫分析法用于现场超灵敏同时检测唾液皮质醇和褪黑激素
现场同时测量皮质醇和褪黑激素对于全面评估昼夜节律和了解生理条件下它们的复杂相互作用是有价值的。然而,目前还没有商业的即时检测能够同时检测这些唾液激素。因此,有一个现场,用户友好的诊断平台的显著需求。虽然侧流免疫分析法(LFI)广泛应用于即时检测,但其灵敏度不足以检测唾液皮质醇和褪黑激素。为了克服这一限制,先前开发的用于唾液皮质醇检测的自扩增LFI (saLFI)被扩展到能够结合金增强方法检测唾液褪黑素。使用双通道样品垫将两个salfi集成到一个设备中,创建双通道自放大侧流免疫测定(2ch-saLFI)。这种集成的双通道样品垫提供了一个均匀分布的样品溶液到两个条带和两个自扩增的配合物。因此,新型2ch-saLFI可以在单次样品注射中同时现场检测两种激素,并在30分钟内提供结果。2ch-saLFI显示出高灵敏度,皮质醇和褪黑激素的检测限分别为5.39和0.476 pg mL−1(即低于典型的生理水平)。使用20个临床样本进行验证,与标准ELISA方法具有很强的相关性(皮质醇的R2 = 0.9017,褪黑激素的R2 = 0.9101)。总的来说,2ch-saLFI代表了第一个通过单次样品注射实现现场,同时和高灵敏度唾液皮质醇和褪黑激素测量的护理点测试。这一发展为先进的医疗保健应用提供了可负担得起的昼夜生物学临床评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


