Reaction kinetics of organic sulfur compounds with ozone in aqueous phase
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Sulfur functional groups are abundant in natural organic compounds, pesticides and pharmaceuticals. Kinetic data of their aqueous reaction with ozone is only available for a very small set of compounds and sometimes contradictory, making predictions on their fate during ozonation processes difficult. Therefore, second-order rate constants kO₃ were determined for 37 sulfur-containing compounds and trends in reactivity were identified. For the determination of kO₃ via competition kinetics, aldicarb was established as pH-independent competitor (kO₃=1.0 × 10⁶M⁻¹s⁻¹). Sulfides (thioethers) react very fast with ozone (kO₃≈2 × 10⁶M⁻¹s⁻¹). Exceptions are sulfides, which are π-conjugated to electron-withdrawing groups (e.g., thiocarbamates, kO₃≈10⁰−10³M⁻¹s⁻¹), and β-lactam antibiotics (kO₃≈10³M⁻¹s⁻¹), which are deactivated to different degrees. Thiols exhibit pH-dependent reactivities, though the dependency is much smaller than previously reported, ranging from kO₃+SH≈104M⁻¹s⁻¹ to kO₃+S⁻≈10⁶M⁻¹s⁻¹, but an unprecedented pH-dependency is observed. A deactivating effect of neighboring groups renders the reactivity of the disulfide cystine and similar compounds pH-dependent (kO₃(pH3)≈10²M⁻¹s⁻¹, kO₃(pH7)≈10⁵M⁻¹s⁻¹) while disulfides without acidic functional groups exhibit pH-independent reactivity (kO₃≈2 × 10⁵M⁻¹s⁻¹). Further oxidized functional groups like sulfoxides or thiosulfinate esters are unreactive (kO₃≈10⁰−10¹M⁻¹s⁻¹), but sulfinates are very susceptible to ozonation (kO₃≈2 × 10⁶M⁻¹s⁻¹). A definitive explanation for this difference is still lacking. For non-N-alkylated isothiazolones, an unexpected pH-dependency is observed, similar to cysteines. The reactivity at pH 7 differs by 4 orders of magnitude, whether or not N-alkylated (kO₃≈10²M⁻¹s⁻¹, kO₃≈10⁶M⁻¹s⁻¹, respectively). Overall, the data presented provides novel insights into the ozone reactivity of sulfur compounds as a basis for a better assessment of their fate during ozonation.
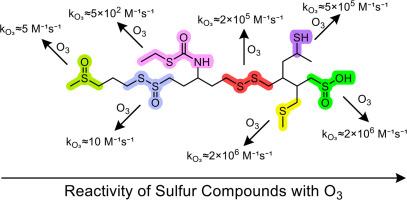
有机硫化合物与臭氧在水相中的反应动力学
硫官能团在天然有机化合物、农药和药物中含量丰富。它们在水中与臭氧反应的动力学数据只适用于极少数化合物,而且有时是相互矛盾的,这使得预测它们在臭氧化过程中的命运变得困难。因此,确定了37种含硫化合物的二级速率常数kO₃,并确定了反应性的趋势。为了通过竞争动力学来确定kO₃的作用,我们建立了甲灭威作为ph无关的竞争对手(kO₃=1.0 × 10⁶M⁻¹s⁻¹)。硫化物(硫醚)与臭氧反应非常快(kO₃≈2 × 10⁶M⁻¹s⁻¹)。但硫化物是例外,它与吸电子基团π共轭(例如硫氨基甲酸盐,kO₃≈10⁰−10³M⁻¹)和β-内酰胺类抗生素(kO₃≈10³M⁻¹),它们在不同程度上被钝化。巯基醇表现出ph依赖性反应,尽管这种依赖性比以前报道的要小得多,从kO₃+SH≈104M⁻⁻到kO₃+ s⁻≈10⁶M⁻⁻,但我们发现了前所未有的ph依赖性。邻近基团的失活作用使得二硫基胱氨酸和类似化合物的反应性与ph有关(kO₃(pH3)≈10²M⁻¹(⁻),kO₃(pH7)≈10个M⁻¹(⁻)),而没有酸性官能团的二硫基则表现出与ph无关的反应性(kO₃≈2 × 10个M⁻¹)。进一步氧化的官能团,如亚砜或硫代亚磺酸酯是不反应的(kO₃≈10⁰−10¹M⁻¹),但是亚磺酸盐非常容易被臭氧化(kO₃≈2 × 10⁶M⁻¹)。对于这种差异,目前仍缺乏一个明确的解释。对于非n-烷基化异噻唑酮,观察到一个意想不到的ph依赖性,类似于半胱氨酸。不管n -烷基化与否,pH值为7的反应性相差4个数量级(kO₃≈10²M⁻¹s⁻,kO₃≈10⁶M⁻¹s⁻)。总的来说,所提供的数据为硫化合物的臭氧反应性提供了新的见解,作为更好地评估它们在臭氧化过程中的命运的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


