Route-dependent toxicodynamics of 6PPD-quinone in mussels: Mechanical resilience trades off with subcellular injury and metabolic disruption
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The byssal thread, a mussel-secreted proteinaceous anchor critical for underwater adhesion, represents a vital adaptation for survival in dynamic marine environments but faces vulnerability to pollutants. This study examines how the tire-derived contaminant 6PPD-quinone (6PPD-Q) impacts the byssal defense system of Mytilus coruscus via waterborne and dietary exposure. Experiments evaluated byssal production, mechanical traits, foot histology, and transcriptomic profiles. Waterborne 6PPD-Q induced a paradoxical enhancement: increased thread count/diameter and adhesion strength coexisted with progressive foot tissue damage, evidenced by histopathology and dysregulated ribosomal/DNA repair pathways. Dietary exposure, conversely, disrupted nutrient metabolism and immune responses, with transcriptomes diverging sharply from waterborne cases. KEGG analysis revealed route-specific toxicity: waterborne exposure activated nuclear DNA damage pathways, while dietary exposure triggered lysosomal/antigen-processing mechanisms. Solvent controls confirmed 6PPD-Q specificity. These findings unveil a dual paradox where 6PPD-Q simultaneously enhances mechanical resilience and inflicts subcellular harm, with toxicodynamics governed by exposure route. The trade-off between structural fortification and physiological impairment highlights complex pollutant interactions in mussels, emphasizing the need for exposure pathway-specific assessments in managing aquaculture sustainability amid coastal contamination. This work advances understanding of anthropogenic pollutant impacts on marine bivalve adaptive strategies and ecosystem health.
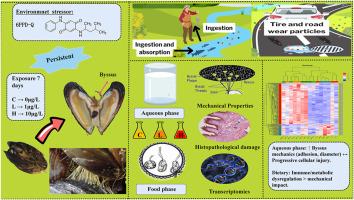
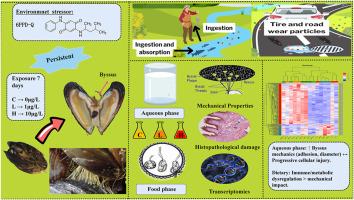
贻贝中6ppd -醌的路线依赖毒性动力学:机械弹性与亚细胞损伤和代谢破坏之间的权衡
足丝是贻贝分泌的一种蛋白质锚,对水下粘附至关重要,是在动态海洋环境中生存的重要适应,但容易受到污染物的影响。本研究探讨了轮胎衍生污染物6ppd -醌(6PPD-Q)如何通过水生和饮食暴露影响贻贝的基底防御系统。实验评估了足部产量、机械性状、足部组织学和转录组特征。水性6PPD-Q诱导了一种矛盾的增强:组织病理学和核糖体/DNA修复途径失调证明,丝线数/直径和粘附强度的增加与进行性足部组织损伤共存。相反,饮食暴露会破坏营养代谢和免疫反应,转录组与水传播病例的差异很大。KEGG分析揭示了途径特异性毒性:水接触激活了核DNA损伤途径,而饮食接触触发了溶酶体/抗原加工机制。溶剂对照证实6PPD-Q特异性。这些发现揭示了一个双重悖论,即6PPD-Q同时增强机械弹性和造成亚细胞伤害,毒性动力学受暴露途径控制。结构强化和生理损伤之间的权衡突出了贻贝中复杂的污染物相互作用,强调了在沿海污染中管理水产养殖可持续性时需要针对暴露途径进行评估。这项工作促进了对人为污染物对海洋双壳类动物适应策略和生态系统健康影响的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


