Exploring the removal of flame retardants and chlorobenzenes by plastic-based materials
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In recent decades, emerging contaminants like chlorobenzenes and flame retardants have raised global concern due to their environmental persistence. However, research into their removal from water remains in its infancy. In this study, pentachlorobenzene (PeCB) and 2,2′,4,4′-tetrabromodiphenyl ether (BDE 47) were selected as representative chlorobenzene and flame retardant compounds, respectively. Adsorption has proven to be a reliable technique for removing emerging contaminants from aquatic environments. For this research, raw and dopamine modified polypropylene ropes (using waste ropes collected from Scottish beaches) were utilized to adsorb PeCB and BDE 47 from water. The raw plastic exhibited a higher adsorption capacity for PeCB, with an adsorption capacity of up to 686 μg/g obtained based on the Langmuir model. However, the modified plastic demonstrated enhanced removal efficiency for BDE 47, with a maximum adsorption capacity of 627 μg/g with the Langmuir model. Both processes were endothermic and spontaneous, and well fitted to Langmuir and pseudo-second-order models. Characterisation analysis revealed PeCB adsorption relied on hydrophobic and π-π interactions, whereas BDE 47 adsorption potentially involved additional n-π interactions and hydrogen bonding. These findings advance understanding of contaminant-plastic interactions in both controlled and natural aquatic environments, offering a sustainable strategy for upcycling fishery waste (i.e., ropes and nets) into functional adsorbents.
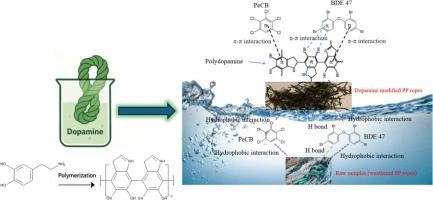
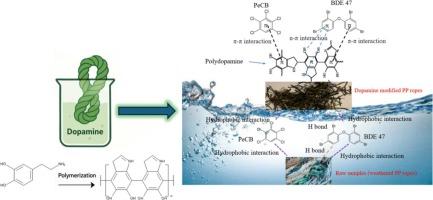
探索用塑料基材料去除阻燃剂和氯苯
近几十年来,氯苯和阻燃剂等新出现的污染物由于其环境持久性引起了全球关注。然而,从水中去除它们的研究仍处于起步阶段。本研究选择五氯苯(PeCB)和2,2 ',4,4 ' -四溴联苯醚(BDE 47)分别作为氯苯和阻燃化合物的代表。吸附已被证明是从水生环境中去除新出现的污染物的可靠技术。在本研究中,使用原料和多巴胺改性聚丙烯绳(使用从苏格兰海滩收集的废绳)来吸附水中的多溴联苯和苯醚47。根据Langmuir模型,原料塑料对PeCB的吸附量可达686 μg/g。然而,改性塑料对BDE 47的去除效率提高,在Langmuir模型下,最大吸附量为627 μg/g。这两个过程都是吸热自发的,并且很好地符合Langmuir和伪二阶模型。表征分析表明,PeCB的吸附依赖于疏水和π-π相互作用,而BDE 47的吸附可能涉及额外的n-π相互作用和氢键。这些发现促进了对受控和自然水生环境中污染物-塑料相互作用的理解,为将渔业废物(即绳索和渔网)升级为功能吸附剂提供了可持续的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


