Liver-breast communication of adipocyte-oriented exosomes drives primary mammary cancer progression
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The incidence of certain types of extrahepatic cancers significantly increases in nonalcoholic fatty liver disease (NAFLD), the mechanisms of which are elusive. Here, we demonstrate that NAFLD is correlated with a higher risk of breast cancer in individuals with atypical hyperplasia and poor prognosis in patients with breast cancer. In mice, fatty liver exosomes are preferentially accumulated in adipocytes, and their enrichment in mammary adipocytes fosters a pro-tumor breast microenvironment. Adipocyte tropism is dictated by the binding of exosomal ErbB4 to neuregulin 4 (Nrg4). tRNA methyltransferase 10 homolog C (TRMT10C) in fatty liver exosomes translocates to mitochondria and inhibits Nd5 and Nd6 mRNA translation by inducing N1-methyladenosine modifications in adipocytes. ND5 and ND6 reduction increases reactive oxygen species and consequently enhances free fatty acid release, which fuels tumor progression. Plasma ErbB4+ exosomes are an independent prognostic factor for patients with breast cancer and comorbid NAFLD. Collectively, we reveal a liver-breast metabolic remote interaction that drives cancer development.
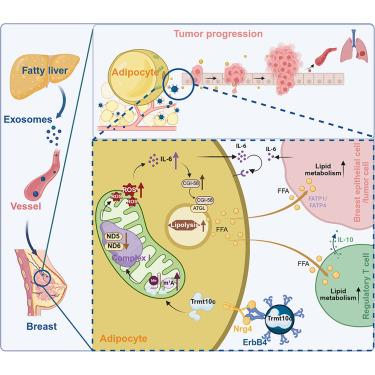
脂肪细胞导向外泌体的肝-乳腺通讯驱动原发性乳腺癌的进展
非酒精性脂肪性肝病(NAFLD)中某些类型肝外癌的发生率显著增加,其机制尚不清楚。在这里,我们证明NAFLD与非典型增生和乳腺癌患者预后差的个体中乳腺癌的高风险相关。在小鼠中,脂肪肝外泌体优先在脂肪细胞中积累,它们在乳腺脂肪细胞中的富集促进了促肿瘤的乳腺微环境。脂肪细胞的趋向性是由外泌体ErbB4与神经调节蛋白4 (Nrg4)的结合决定的。脂肪肝外泌体tRNA甲基转移酶10同源物C (TRMT10C)易位至线粒体,通过诱导脂肪细胞中n1 -甲基腺苷修饰抑制Nd5和Nd6 mRNA的翻译。ND5和ND6的减少增加了活性氧,从而增加了游离脂肪酸的释放,从而促进了肿瘤的进展。血浆ErbB4+外泌体是乳腺癌合并NAFLD患者的独立预后因素。总的来说,我们揭示了肝脏-乳房代谢的远程相互作用,驱动癌症的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


