Astrocytes functionally integrate multiple synapses via specialized leaflet domains
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Astrocyte Ca2+ dynamics control synaptic circuits and behavior, yet the underlying biology remains poorly understood. By combining volumetric high-resolution electron microscopy and two-photon Ca2+ imaging, we characterize astrocyte leaflets that interface with synapses. These convoluted structures with ≤250 nm diameter originate from astrocytic shafts or cell bodies, contain minuscule endoplasmic reticulum saccules expressing IP3 receptors but not mitochondria, and are often interconnected via gap junctions forming domains with cytosolic continuity. Leaflets enwrap 90% of synapses in clusters and only 10% individually. By fast imaging of astrocyte peripheral microvolumes, we identify leaflet-specific Ca2+ events that were synaptically induced, IP3R1-mediated, and often displayed separate originations merging into large, long-lasting Ca2+ elevations. Using combined axon-leaflet Ca2+ imaging, we show that these complex events reflect integration of incoming inputs from different neurons. The astrocyte leaflet organization may thus coordinate, via Ca2+ signals, multiple synapses and circuits active at different spatiotemporal scales, executing computations distinct from neurons.
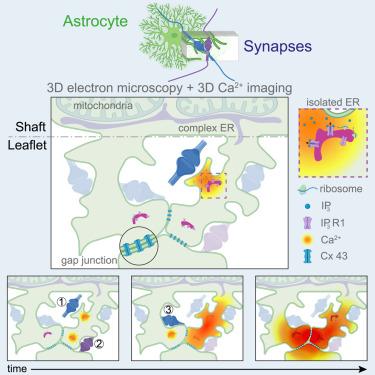
星形胶质细胞通过专门的小叶结构域整合多个突触
星形胶质细胞Ca2+动力学控制突触回路和行为,但潜在的生物学仍然知之甚少。通过结合体积高分辨率电子显微镜和双光子Ca2+成像,我们表征了与突触界面的星形胶质细胞小叶。这些直径≤250nm的卷曲结构来自星形细胞轴或细胞体,包含表达IP3受体但不含线粒体的微小内质网小囊,并且通常通过间隙连接形成具有细胞质连续性的结构域相互连接。小叶包裹着90%的突触簇,只有10%的突触单独包裹。通过星形胶质细胞外周微体积的快速成像,我们确定了突触诱导的,ip3r1介导的叶片特异性Ca2+事件,并且经常显示单独的起源合并成大的,持久的Ca2+升高。使用联合轴突-小叶Ca2+成像,我们表明这些复杂的事件反映了来自不同神经元的传入输入的整合。星形胶质细胞小叶组织可能因此协调,通过Ca2+信号,多个突触和电路在不同的时空尺度上活跃,执行不同于神经元的计算。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


