Iron-catalysed radical Markovnikov hydroamidation of complex alkenes
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
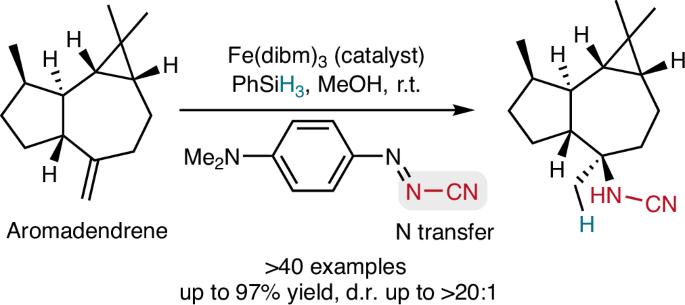
Nitrogen atoms are integral components of various chemical functional groups, including amines, amides and N-heterocycles, among others. Consequently, they play an important role in pharmaceuticals, agrochemicals, natural products, materials and commodity chemicals. The formation of C–N bonds is reliably achieved through methods, such as reductive amination, N-alkylation and cross-coupling. Hydroamination, starting with alkenes, presents a valuable alternative for accessing organic compounds containing nitrogen, as alkenes are highly abundant. Here we present a method for the iron-catalysed radical hydroamidation of alkenes. To this end, we developed a radical amidation reagent that can be readily prepared on a large scale, facilitating the efficient transfer of the synthetically valuable cyanamide functionality across both activated and unactivated double bonds. The scope of the reaction is remarkably broad, demonstrating its applicability to the diastereoselective hydroamidation of complex terpene natural products. Importantly, the synthesis of 15N-labelled amines is possible using this strategy. Subsequent chemistry, converting the distinctive cyanamide functionality into other useful groups, further validates the value of the developed methodology. An iron-catalysed radical Markovnikov hydroamidation of alkenes using a cyanamide reagent is reported. The method achieves C–N bond formation with high yields and selectivity and is applicable to a wide range of alkenes and natural products. The cyanamide functionality can be transformed into various functional groups, highlighting its potential for advanced applications in natural product synthesis.
铁催化的络合烯烃自由基马尔可夫尼科夫氢酰胺化反应
氮原子是各种化学官能团的组成部分,包括胺、酰胺和n -杂环等。因此,它们在药品、农用化学品、天然产品、材料和商品化学品中发挥着重要作用。通过还原胺化、n -烷基化和交叉偶联等方法可靠地形成C-N键。从烯烃开始的氢胺化反应为获取含氮有机化合物提供了一种有价值的替代方法,因为烯烃非常丰富。本文提出了一种铁催化烯烃自由基氢酰胺化反应的方法。为此,我们开发了一种易于大规模制备的自由基酰胺化试剂,促进了合成有价值的氰酰胺功能在活化和非活化双键上的有效转移。反应范围非常广泛,表明其适用于复杂萜烯天然产物的非对映选择性加氢酰胺化。重要的是,使用这种策略可以合成15n标记的胺。随后的化学反应,将独特的氰酰胺功能转化为其他有用的基团,进一步验证了所开发方法的价值。报道了一种用氰胺试剂铁催化烯烃自由基马尔可夫尼科夫氢酰胺化反应。该方法生成的C-N键收率高,选择性好,适用于多种烯烃和天然产物。氰酰胺的功能可以转化为各种官能团,突出了其在天然产物合成中的先进应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


