Unassisted electrochemical H2O2 production coupled to glycerol oxidation
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
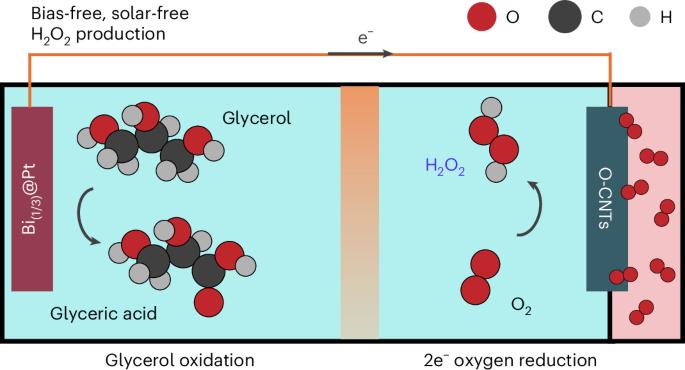
Hydrogen peroxide (H2O2) is not only a key eco-friendly oxidizer but also a promising energy carrier with an energy density comparable to that of compressed hydrogen. The industrial production of H2O2 relies on the energy-intensive and environmentally detrimental anthraquinone process, necessitating the exploration of greener alternatives. Here we demonstrate sustainable and unassisted electrochemical H2O2 production (via the two-electron oxygen reduction reaction) coupled to the oxidative valorization of glycerol, a biomass energy by-product, operating without external electric or solar energy inputs. We applied bismuth-loaded Pt and oxidized carbon nanotube electrocatalysts, for glycerol oxidation reaction and two-electron oxygen reduction reaction, respectively, which possess onset potentials close to the theoretical values for the electrochemical reactions. With this system, we achieved a high H2O2 production rate of approximately 8.475 μmol cm−2 min−1 and high glycerate selectivity for in situ glycerol oxidation reaction (74%), while producing renewable electricity on-site. Hydrogen peroxide (H2O2) is an eco-friendly oxidizer and a promising energy carrier, but it is primarily synthesized through the energy-intensive anthraquinone process. Here sustainable and unassisted electrochemical H2O2 production coupled to glycerol oxidation is reported, operating without an external bias or solar energy input.
与甘油氧化耦合的无辅助电化学H2O2生产
过氧化氢(H2O2)不仅是一种重要的生态友好型氧化剂,而且具有与压缩氢相当的能量密度,是一种很有前途的能量载体。H2O2的工业生产依赖于能源密集型和对环境有害的蒽醌工艺,因此需要探索更环保的替代品。在这里,我们展示了可持续和无辅助的电化学H2O2生产(通过双电子氧还原反应)与甘油(一种生物质能源副产品)的氧化增值相结合,在没有外部电力或太阳能输入的情况下运行。我们将负载铋的铂电催化剂和氧化碳纳米管电催化剂分别应用于甘油氧化反应和双电子氧还原反应,其起始电位接近电化学反应的理论值。利用该系统,我们实现了约8.475 μmol cm−2 min−1的高H2O2产率和高甘油原位氧化反应的选择性(74%),同时在现场产生可再生电力。过氧化氢(H2O2)是一种生态友好的氧化剂,也是一种很有前途的能量载体,但它主要是通过高耗能的蒽醌法合成的。在这里,报告了可持续的、无辅助的电化学H2O2生产与甘油氧化耦合,在没有外部偏压或太阳能输入的情况下运行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


