Geometric distortion as an enabling tool for organic synthesis
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
π-bonds are typically associated with well-defined arrangements of atoms. However, when the arrangement of atoms associated with these bonds becomes geometrically distorted, heightened reactivity is seen, enabling a wide range of transformations that can proceed under mild reaction conditions. As a result, molecules bearing complex structures can be rapidly assembled from simple building blocks. Here we describe the strategic use of synthetic building blocks containing π-bonds that feature geometric distortion, with a focus on recent applications to organic synthesis. The specific building blocks discussed are arynes, cyclic allenes, cyclic 1,2,3-trienes and anti-Bredt olefins. Developments in transition metal-mediated chemistry that enable previously unknown transformations are discussed, as well as new strategies for complex molecule and natural product synthesis that take advantage of geometrically distorted intermediates. We hope this Review will inspire future advances in the strategic use of geometric distortion in chemical synthesis. Although π-bonds are typically associated with having well-defined arrangements of atoms, ring constraints can lead to geometric distortion, resulting in heightened reactivity. These effects can be leveraged to enable synthetic transformations. This Review features processes wherein geometric distortion is leveraged to provide rapid access to structurally complex products.
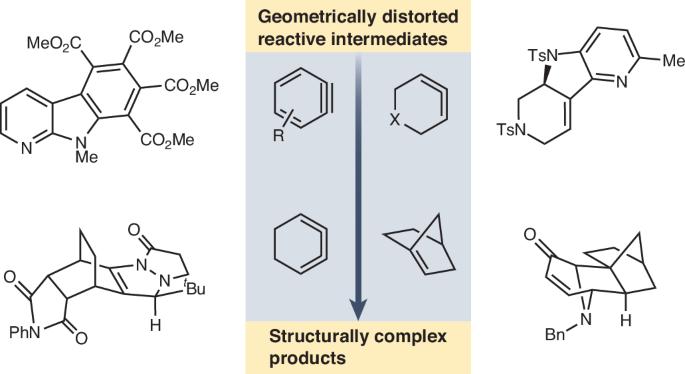
几何畸变是有机合成的有利工具
π键通常与明确的原子排列有关。然而,当与这些键相关的原子排列发生几何扭曲时,可以看到反应性增强,从而可以在温和的反应条件下进行大范围的转化。因此,具有复杂结构的分子可以从简单的构建块快速组装起来。在这里,我们描述了战略性地使用含有π键的合成构件,其特征是几何畸变,重点是最近在有机合成中的应用。具体讨论了芳烃、环烯、环1,2,3-三烯和抗bredt烯烃。讨论了过渡金属介导化学的发展,使以前未知的转化成为可能,以及利用几何扭曲的中间体合成复杂分子和天然产物的新策略。我们希望这篇综述能启发几何畸变在化学合成中的战略性应用。虽然π键通常与具有明确定义的原子排列有关,但环约束可能导致几何畸变,从而导致反应性提高。可以利用这些效果来启用合成转换。这篇综述的特点是利用几何扭曲来快速获取结构复杂的产品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


