On-surface synthesis and characterization of linear and cyclic C6
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
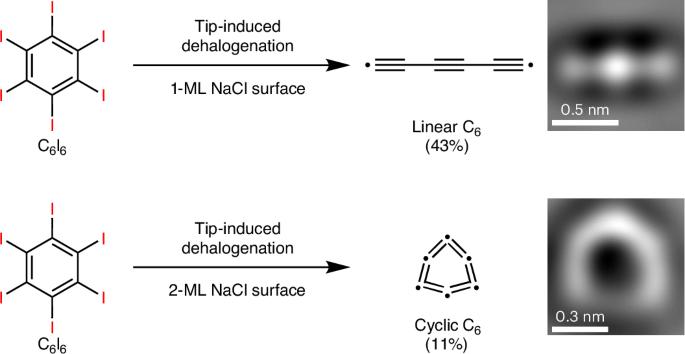
Cyclo[n]carbons, belonging to carbon clusters (Cn), have attracted substantial attention from both experimentalists and theoreticians because of their chemical structures and the controversial stabilities of their different isomers. Recently, C26, C20, C18, C16, C14, C13, C12 and C10 have been synthesized and characterized on thin insulating NaCl surfaces. However, synthesis of smaller cyclocarbons (n < 10) remains challenging due to their inherent high reactivity and increased strain. In particular, C6 has been a subject of long-standing theoretical debate because the energy difference between its linear and cyclic forms is very small. Here we successfully generate both linear and cyclic C6 by modulating the thickness of NaCl layers deposited on a Au(111) surface, using tip-induced dehalogenation of hexaiodobenzene (C6I6) molecules at 4.7 K. The linear C6 was generated on 1-monolayer (1-ML) or 2-ML NaCl surfaces and identified as a polyynic structure through bond-resolved atomic force microscopy, verifying the theoretical calculations of a Peierls transition for linear C6 from gas phase to on-surface adsorption. Meanwhile, cyclic C6 could be generated on a 2-ML NaCl surface and its cumulenic nature is confirmed both experimentally and theoretically. Additionally, voltage pulses can induce cyclic C6 to undergo either an adsorption configuration transformation from a nearly planar to a tilted geometry, or a structural transformation to the linear form. Linear C6 and its isomer, cyclic C6 (the smallest aromatic cyclocarbon), are successfully generated on NaCl surfaces by tip-induced dehalogenation of hexaiodobenzene molecules at 4.7 K, and their polyynic and cumulenic structures, respectively, are characterized by bond-resolved atomic force microscopy.
线性和环状C6的表面合成与表征
环[n]碳属于碳簇(Cn),由于其化学结构和其不同异构体的有争议的稳定性,引起了实验家和理论家的大量关注。近年来合成了C26、C20、C18、C16、C14、C13、C12和C10,并在薄绝缘NaCl表面上进行了表征。然而,由于其固有的高反应性和增加的应变,较小的环碳化合物(n < 10)的合成仍然具有挑战性。特别是,C6一直是一个长期存在的理论争论的主题,因为它的线性和循环形式之间的能量差非常小。本研究通过调节沉积在Au(111)表面的NaCl层的厚度,在4.7 K的温度下,利用尖端诱导的六碘苯(C6I6)分子脱卤,成功地生成了线性和环状C6。线性C6在1-单层(1-ML)或2-ML NaCl表面生成,并通过键分辨原子力显微镜鉴定为多聚结构,验证了线性C6从气相到表面吸附的Peierls转变的理论计算。同时,在2-ML NaCl表面可以生成环状C6,并从实验和理论上证实了其积累性。此外,电压脉冲可以诱导环C6经历从近平面到倾斜几何的吸附构型转变,或结构转变为线性形式。在4.7 K的温度下,通过尖端诱导六氯苯分子脱卤,在NaCl表面成功生成了线性C6及其同分异构体环C6(最小的芳香族环碳),并用键分辨原子力显微镜对其多聚和累积结构进行了表征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


