Stereoconvergent reduction of alkenes using a repurposed iron-based dioxygenase
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The stereoconvergent reduction of alkenes for efficient synthesis of chiral compounds is a challenge in synthetic chemistry, even for exquisite enzymes in nature. Natural ene-reductases for example generally catalyse the reduction of alkenes in an enantiodivergent or resolution fashion. Here we report the repurposing of non-haem iron-based dioxygenases to catalyse the stereoconvergent reduction of alkenes through an iron hydride intermediate, by introducing silanes to the biocatalytic system. Directed evolution of gentisate 1,2-dioxygenase led to iron-based ene-reductases with high efficiency (up to 99% yield), excellent enantioselectivity (23 examples with >99% e.e.) and compatibility with a structurally diverse range of substrates. Experimental studies suggest the formation of iron hydride species in the enzyme and support the divalency of iron during the catalytic process. Computational studies show that the reaction is energetically feasible through an iron hydride mechanism and reveal the molecular mechanism of stereoconvergence. A repurposed non-haem, iron-based dioxygenase enables the stereoconvergent reduction of alkenes with excellent selectivity. Mechanistic studies support an iron hydride pathway and reveal the molecular mechanism of stereoconvergence.
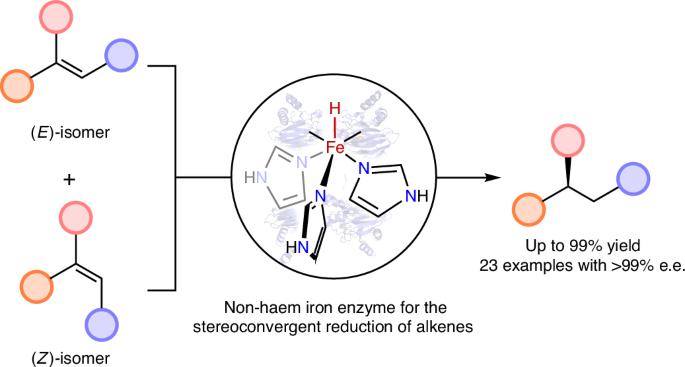
利用改造的铁基双加氧酶进行烯烃的立体聚合还原
对烯烃进行立体会聚还原以有效合成手性化合物是合成化学中的一个挑战,即使对自然界中精细的酶也是如此。例如,天然的烯还原酶通常以对映发散或分解的方式催化烯烃的还原。在这里,我们报告了非血红素铁基双加氧酶的重新用途,通过将硅烷引入生物催化系统,通过氢化铁中间体催化烯烃的立体收敛还原。龙骨酸1,2-双加氧酶的定向进化导致铁基烯还原酶具有高效率(高达99%的产率),优异的对映体选择性(23个例子具有99%的e.e)和与多种结构底物的相容性。实验研究表明,在催化过程中,氢氧化铁在酶中形成,并支持铁的二价性。计算研究表明,该反应在能量上是可行的,并揭示了立体会聚的分子机理。一个重新利用的非血红素,铁基双加氧酶使立体收敛还原烯烃具有极好的选择性。机理研究支持氢化铁途径,揭示了立体会聚的分子机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


