Unravelling the structure-dependent defluorination mechanisms of per- and polyfluoroalkyl substances by hydrated electrons in UV/sulfite
IF 24.1
引用次数: 0
Abstract
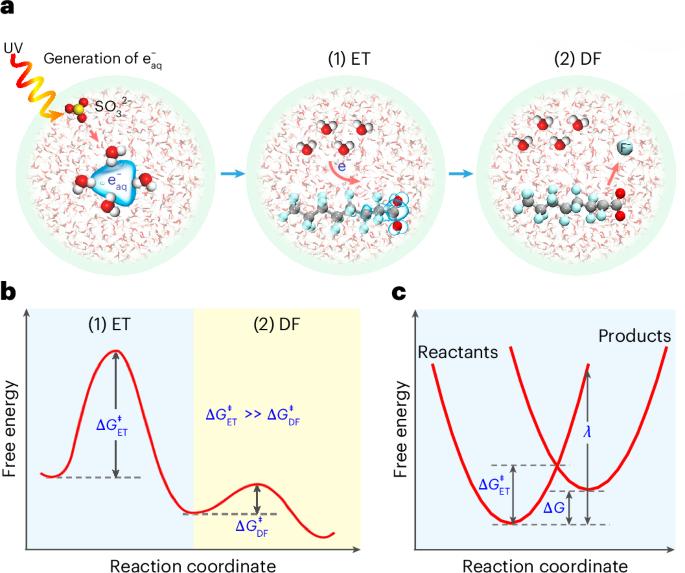
Hydrated electron ( $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ )-based technologies are promising for resolving the global pollution of per- and polyfluoroalkyl substances (PFAS) by efficiently breaking C−F bonds. However, achieving complete defluorination has been challenging, and the fundamental mechanisms behind the stalled efficacy and varied reactivity remain obscure. Here we propose an electron transfer (ET)-limiting mechanism for PFAS degradation by $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ through experimental and theoretical evaluations of 41 structures in UV/sulfite. The degradation rate constants spanned four orders of magnitude, with 34 PFAS achieving ~100% defluorination. We found that the defluorination occurs in a stepwise manner, with ET from $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ to PFAS being rate limiting rather than subsequent C−F bond cleavage. This ET-limiting mechanism was verified by the effectiveness of the free energy of activation (2.33–27.4 kcal mol−1) calculated on the basis of the Marcus theory to predict the distinct reactivity of PFAS. Combined with spin-density analysis, this mechanism reveals that C=C, C−Cl, CF2COO− and (CF2)n≥6 promote the complete defluorination by favouring ET, whereas ET-disfavouring moieties, C−H, −O−, (CH2)n, SO3− and (CF2)n≤3, hinder the defluorination to varying extents. In particular, most PFAS were found to undergo initial attack of $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ either at α-CF2 of CF2COO− or in the middle of (CF2)n≥6, resulting in two defluorination patterns with extensive or absent intermediates, respectively. The ET-limiting mechanism captures these different pathways that align with experimental results by governing the reactivity of potential intermediates. Our theoretical framework explains the $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ -induced defluorination process and provides insights into designing rapid degradable substitutes to tackle the PFAS crisis. Complete defluorination of diverse per- and polyfluoroalkyl substances can be achieved in the UV/sulfite system. Further experimental and theoretical evaluation demonstrates that the rate-limiting step is electron transfer from hydrated electrons, rather than the subsequent C−F bond cleavage.
在UV/亚硫酸盐中水合电子揭示全氟和多氟烷基物质结构依赖的脱氟机制
基于水合电子($${\mathrm{e}}_{{\rm{aq}}}^{-}$$)的技术有望通过有效破坏C−F键来解决全氟烷基和多氟烷基物质(PFAS)的全球污染。然而,实现完全除氟一直具有挑战性,而功效停滞和反应性变化背后的基本机制仍然不清楚。本文通过对紫外光/亚硫酸盐中41种结构的实验和理论评价,提出了$${\mathrm{e}}_{{\rm{aq}}}^{-}$$降解PFAS的电子转移(ET)限制机制。降解速率常数跨越4个数量级,34个PFAS达到100% defluorination. We found that the defluorination occurs in a stepwise manner, with ET from $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ to PFAS being rate limiting rather than subsequent C−F bond cleavage. This ET-limiting mechanism was verified by the effectiveness of the free energy of activation (2.33–27.4 kcal mol−1) calculated on the basis of the Marcus theory to predict the distinct reactivity of PFAS. Combined with spin-density analysis, this mechanism reveals that C=C, C−Cl, CF2COO− and (CF2)n≥6 promote the complete defluorination by favouring ET, whereas ET-disfavouring moieties, C−H, −O−, (CH2)n, SO3− and (CF2)n≤3, hinder the defluorination to varying extents. In particular, most PFAS were found to undergo initial attack of $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ either at α-CF2 of CF2COO− or in the middle of (CF2)n≥6, resulting in two defluorination patterns with extensive or absent intermediates, respectively. The ET-limiting mechanism captures these different pathways that align with experimental results by governing the reactivity of potential intermediates. Our theoretical framework explains the $${\mathrm{e}}_{{\rm{aq}}}^{-}$$ -induced defluorination process and provides insights into designing rapid degradable substitutes to tackle the PFAS crisis. Complete defluorination of diverse per- and polyfluoroalkyl substances can be achieved in the UV/sulfite system. Further experimental and theoretical evaluation demonstrates that the rate-limiting step is electron transfer from hydrated electrons, rather than the subsequent C−F bond cleavage.
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


