Electron-buffering rechargeable microelectrode adsorbents for rapid environmental remediation of uranium-containing wastewater
IF 24.1
引用次数: 0
Abstract
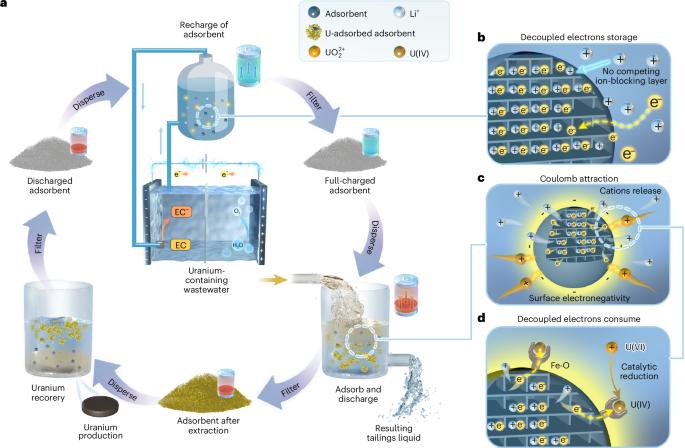
Environmental remediation of uranium-containing wastewater has emerged as a critical challenge in the nuclear industry, addressing both ecological protection and resource sustainability concerns. However, conventional adsorption methods suffer from slow kinetics, whereas electrochemical approaches are limited by the direct coupling between electron injection and uranyl ion reduction, leading to ion-blocking layers that severely impede remediation efficiency. Here we develop an electron-buffering rechargeable microelectrode adsorbent system that operates through a three-step cycle: electron storage, uranium extraction and adsorbent regeneration. This sequential process decouples electron injection from uranyl ion reduction by separating them in time and space. The microelectrode stores electrons in an environment free of competing ions and releases them in a controlled manner during uranium extraction. The Fe–O bonds on the surface serve as active sites for capturing uranyl ions and lowering their reduction overpotential, whereas the release of charge-balancing cations maintains surface electronegativity for efficient mass transfer. This synergistic integration achieves an initial extraction rate of 1,062 mg g−1 h−1 and a uranium capacity of 854 mg g−1, with nearly 100% electron utilization efficiency. Most importantly, when tested with actual uranium mine wastewater containing 0.545 ppm uranium, the system achieves 97.1% extraction efficiency and a capacity of 78.5 mg g−1 within 6 h, demonstrating its practical viability for environmental remediation. A synergistic approach for uranium extraction, a rechargeable microelectrode adsorbent system, integrates the functions of adsorption and electrochemical reduction, leading to an excellent extraction rate and high adsorption capacity.
用于含铀废水快速环境修复的电子缓冲可充电微电极吸附剂
含铀废水的环境修复已成为核工业面临的一个严峻挑战,既要解决生态保护问题,又要解决资源可持续性问题。然而,传统的吸附方法动力学缓慢,而电化学方法受限于电子注入和铀酰离子还原之间的直接耦合,导致离子阻断层严重阻碍了修复效率。在这里,我们开发了一种电子缓冲可充电微电极吸附剂系统,该系统通过三步循环:电子存储,铀提取和吸附剂再生。这个顺序过程通过在时间和空间上分离铀酰离子还原来分离电子注入。微电极将电子储存在没有竞争离子的环境中,并在铀提取过程中以受控的方式释放电子。表面的Fe-O键作为捕获铀酰离子并降低其还原过电位的活性位点,而电荷平衡阳离子的释放维持表面电负性以实现有效的传质。这种协同整合实现了初始提取率为1062 mg g−1 h−1,铀容量为854 mg g−1,电子利用效率接近100%。最重要的是,在含铀0.545 ppm的实际铀矿废水中进行试验,该系统在6 h内的萃取效率达到97.1%,萃取量为78.5 mg g−1,证明了该系统在环境修复中的实际可行性。一种协同提取铀的方法——可充电微电极吸附系统,集吸附和电化学还原功能于一体,具有优异的提取率和较高的吸附容量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


