Siderophores as a selective regulator for enhancing anaerobic ammonium oxidation bacteria
IF 24.1
引用次数: 0
Abstract
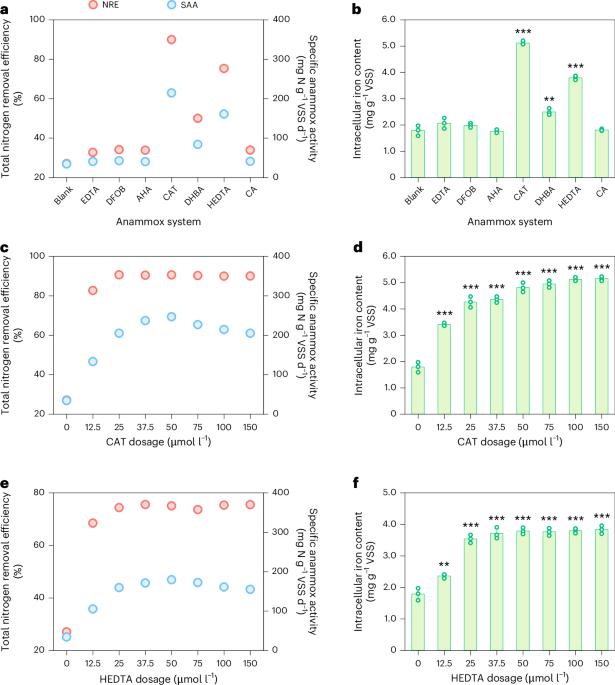
The limited iron uptake efficiency of anaerobic ammonium oxidation (anammox) bacteria has garnered accumulating concerns, as iron plays an important role in anammox bacteria metabolism. Siderophores are a prevalent strategy for iron acquisition among many bacteria, and recent evidence has suggested the potential role of siderophore-Fe3+ as bioavailable iron sources to anammox bacteria. Nonetheless, what target siderophore anammox bacteria can use and how they use it remain poorly understood. Here we shed fresh light on siderophore selectively promoting iron uptake and regulating the metabolic activity of anammox bacteria. Batch experiments and long-term operations demonstrated that siderophores catechin (CAT) and N-hydroxyethyl ethylenediamine triacetic acid (HEDTA) increased the iron uptake efficiency of anammox consortia up to 50%–65% and triggered the cofactors synthesis for key enzymes assembly, thereby selectively enhancing the anammox activity (≥350 mg N per gram of volatile suspended solids per day) and nitrogen removal efficiency (≥85%). Integrated with multi-omics approaches, we revealed versatile iron uptake pathways within anammox bacteria genera: Candidatus Brocadia acquired CAT-Fe3+ and HEDTA-Fe3+ via outer membrane receptors FitA/TbpA and FecA, respectively, whereas Candidatus Jettenia initially reduced CAT-Fe3+ using NfnB enzyme before uptake via the FeoABC system. This work reveals a pivotal role of siderophores in selectively enhancing anammox bacteria by optimizing their iron uptake and utilization and uncovers the involved molecular mechanisms, which opens promising avenues to optimize the anammox process with siderophore regulation for more efficient and sustainable wastewater treatment. The functional bacteria in the anaerobic ammonium oxidation process rely heavily on iron for their metabolism. The addition of exogenous siderophores considerably enhanced the iron uptake efficiency of anammox consortia, leading to enhanced anammox activity and nitrogen removal performance.
铁载体作为增强厌氧氨氧化细菌的选择性调节剂
由于铁在厌氧氨氧化(anammox)细菌的代谢中起着重要作用,因此厌氧氨氧化(anammox)细菌对铁的吸收效率有限引起了人们的关注。铁载体是许多细菌获取铁的一种普遍策略,最近的证据表明铁载体- fe3 +作为厌氧氨氧化细菌的生物可利用铁源的潜在作用。尽管如此,铁载体厌氧氨氧化细菌可以利用什么目标以及它们如何利用它仍然知之甚少。本研究为铁载体选择性促进厌氧氨氧化菌的铁吸收和调节代谢活性提供了新的思路。批量实验和长期操作表明,铁载体儿茶素(CAT)和N-羟乙基乙二胺三乙酸(HEDTA)可使厌氧氨氧化菌群的铁吸收效率提高50%-65%,并触发关键酶组合的辅因子合成,从而选择性地提高厌氧氨氧化活性(≥350 mg N / g / g / g / d)和脱氮效率(≥85%)。结合多组学方法,我们揭示了厌氧氨氧化菌属的多种铁摄取途径:Candidatus Brocadia分别通过外膜受体FitA/TbpA和FecA获得CAT-Fe3+和HEDTA-Fe3+,而Candidatus Jettenia在通过FeoABC系统摄取之前首先使用NfnB酶还原CAT-Fe3+。本研究揭示了铁载体通过优化厌氧氨氧化细菌对铁的吸收和利用,在选择性增强厌氧氨氧化细菌中的关键作用,并揭示了其中的分子机制,为优化厌氧氨氧化过程提供了有希望的途径,通过铁载体调控来实现更高效和可持续的废水处理。厌氧氨氧化过程中的功能细菌严重依赖铁进行代谢。外源铁载体的加入大大提高了厌氧氨氧化菌对铁的吸收效率,从而提高了厌氧氨氧化活性和脱氮性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


