Spin crossover-driven diiron electrocatalyst boosts sustainable water oxidation
IF 27.1
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
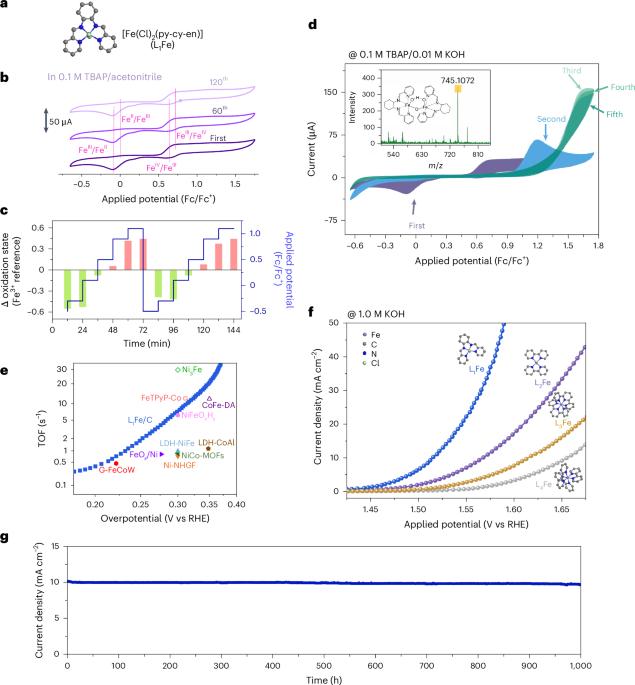
Electrocatalytic reduction of carbon dioxide and water oxidation are promising technologies to mitigate environmental problems. A critical bottleneck, however, is the significant energy loss that arises from the anodic oxygen evolution reaction (OER) with its sluggish kinetics and reliance on scarce noble metals. It is therefore essential to develop earth-abundant and efficient OER catalysts. Here we report the reactive diiron electrocatalyst [Fe2(µ-O)(µ-OH)(L1)2], where L1 is a nitrogen-based ligand, which exhibits an outstanding performance—achieving a turnover frequency of 20.2 s−1 at 1.580 V and a low overpotential of 184 mV at a current density of 10 mA cm−2—and exceptional stability over 1,000 h. This diiron electrocatalyst is formed via a spin crossover-driven dimerization mechanism, where the resulting diiron atomic configuration promotes strong metal–ligand covalency and facilitates the formation of key intermediates that are essential for efficient OER catalysis. Our findings offer a promising strategy for the design of high-performance catalysts for water oxidation and sustainable electrocatalysis. This work shows an iron molecular catalyst for water oxidation, a critical reaction for renewable energy technologies. The excellent performance of the catalyst is attributed to an intermediate dinuclear structure formed via spin crossover.
自旋交叉驱动的双铁电催化剂促进水的可持续氧化
电催化还原二氧化碳和水氧化是缓解环境问题的有前途的技术。然而,一个关键的瓶颈是阳极析氧反应(OER)由于其缓慢的动力学和对稀有贵金属的依赖而产生的巨大能量损失。因此,开发地球资源丰富且高效的OER催化剂至关重要。本文报道了反应性双铁电催化剂[Fe2(µ-O)(µ-OH)(L1)2],其中L1是氮基配体,在1.580 V时具有20.2 s−1的转换频率,在电流密度为10 mA cm−2时具有184 mV的过电位,并且在1,000 h内具有出色的稳定性。这种双铁电催化剂是通过自旋交叉驱动的二聚化机制形成的,其中产生的双铁原子构型促进了强金属配体共价,并促进了高效OER催化所必需的关键中间体的形成。我们的发现为设计用于水氧化和可持续电催化的高性能催化剂提供了一个有希望的策略。这项工作展示了一种用于水氧化的铁分子催化剂,这是可再生能源技术的关键反应。催化剂的优异性能归功于自旋交叉形成的中间双核结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Sustainability
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
41.90
自引率
1.10%
发文量
159
期刊介绍:
Nature Sustainability aims to facilitate cross-disciplinary dialogues and bring together research fields that contribute to understanding how we organize our lives in a finite world and the impacts of our actions.
Nature Sustainability will not only publish fundamental research but also significant investigations into policies and solutions for ensuring human well-being now and in the future.Its ultimate goal is to address the greatest challenges of our time.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


