Electrode separation via water electrolysis for sustainable battery recycling
IF 27.1
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
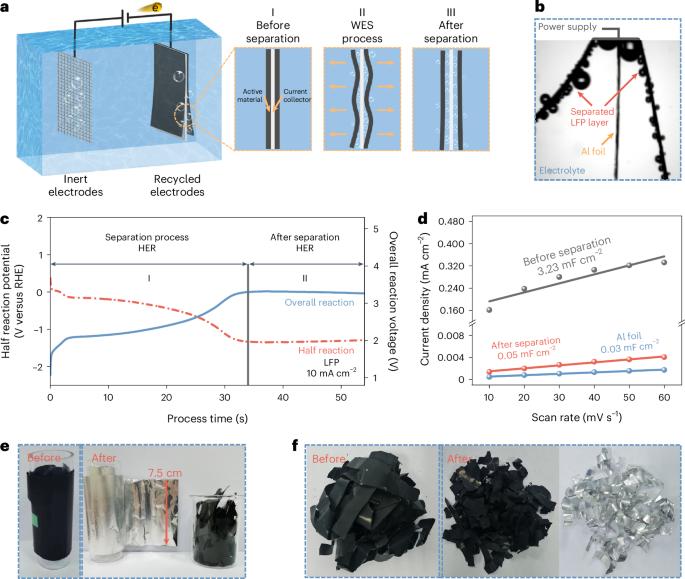
Recycling large quantities of lithium-ion batteries facing retirement is pivotal for resource conservation and environmental sustainability. Direct recycling, while offering a promising avenue with reduced waste compared with pyrometallurgy and hydrometallurgy, often involves intricate and long processes. Here we introduce a water electrolysis-induced separation approach, using H2 or O2 gas bubbling to efficiently separate electrode materials from current collectors. The process achieves 99.5% materials recovery with metal impurities <40 ppm within 34 s for LiFePO4 and 3 s for graphite at 10 mA cm−2, with minimal energy consumption of 11 and 1.1 kJ kgcell−1. Moreover, this approach accommodates various electrode types, encompassing cathodes and anodes from spent batteries or manufacturing scraps. The subsequent dry electrode manufacturing process with lithium replenishment substantially enhances environmental sustainability by eliminating the use of N-methyl pyrrolidone, while maintaining performance through the effective mixing of active materials and conductive agents. An EverBatt analysis underscores a remarkable reduction in energy consumption and waste generation compared with industrially adopted recycling methods. This finding provides an efficient and sustainable solution for battery recycling while ensuring high-quality materials production. Current battery recycling processes face sustainability challenges. Using gas evolution in water electrolysis, this work realizes fast separation of active electrode materials from current collectors before their dry refabrication for electrodes without compromising performance.
通过电解水分离电极,实现电池的可持续回收
回收大量面临退役的锂离子电池对资源节约和环境可持续性至关重要。与火法冶金和湿法冶金相比,直接回收虽然提供了减少废物的有前途的途径,但往往涉及复杂而漫长的过程。在这里,我们介绍了一种水电解诱导分离方法,利用H2或O2气体鼓泡有效地分离电极材料和集流器。在10 mA cm−2的条件下,LiFePO4和石墨在34 s和3 s内实现了99.5%的材料回收率,金属杂质为40 ppm,能耗最小为11 kJ kgcell−1和1.1 kJ kgcell−1。此外,这种方法适用于各种电极类型,包括来自废电池或制造废料的阴极和阳极。随后的锂补充干电极制造工艺通过消除n -甲基吡咯烷酮的使用,大大提高了环境的可持续性,同时通过有效混合活性材料和导电剂保持性能。everbat的分析强调,与工业采用的回收方法相比,能源消耗和废物产生显著减少。这一发现为电池回收提供了一种高效、可持续的解决方案,同时确保了高质量的材料生产。当前的电池回收过程面临着可持续性挑战。利用水电解中的气体演化,这项工作实现了活性电极材料在电极干式再制造之前与集电器的快速分离,而不会影响其性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Sustainability
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
41.90
自引率
1.10%
发文量
159
期刊介绍:
Nature Sustainability aims to facilitate cross-disciplinary dialogues and bring together research fields that contribute to understanding how we organize our lives in a finite world and the impacts of our actions.
Nature Sustainability will not only publish fundamental research but also significant investigations into policies and solutions for ensuring human well-being now and in the future.Its ultimate goal is to address the greatest challenges of our time.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


