The Effect of Proton Donors on the Process of Electrochemical Generation of Thiocyanogen and Its Reactivity
IF 0.8
4区 工程技术
Q4 ELECTROCHEMISTRY
引用次数: 0
Abstract
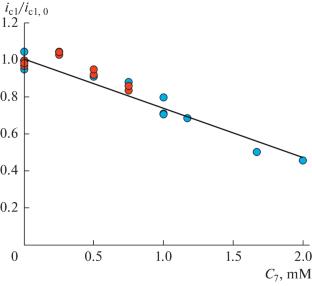
In continuation of our systematic study of the mechanism of thiocyanogen generation via electrochemical oxidation of thiocyanate anion in acetonitrile, the influence of proton donors both on the generation and on the reactivity of thiocyanogen itself was studied by experimental (cyclic voltammetry, electrolysis) and theoretical (digital simulations, quantum chemical calculations) methods. In a series of acids of various strengths (acetic, trifluoroacetic and perchloric acids), the effect was revealed only in the case of a strong proton donor, perchloric acid. It was shown that perchloric acid forms with thiocyanogen a hydrogen-bonded complex, less active in the thiocyanation reaction.
质子供体对硫氰酸根电化学生成过程及其反应活性的影响
为了继续系统研究硫氰酸阴离子在乙腈中电化学氧化生成硫氰酸根的机理,我们通过实验(循环伏安法、电解法)和理论(数字模拟、量子化学计算)方法研究了质子供体对硫氰酸根生成和对硫氰酸根本身反应性的影响。在一系列不同强度的酸(乙酸、三氟乙酸和高氯酸)中,只有在强质子供体高氯酸的情况下才显示出这种效应。结果表明,高氯酸与硫氰化物形成氢键配合物,在硫氰化反应中活性较低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Russian Journal of Electrochemistry
工程技术-电化学
CiteScore
1.90
自引率
8.30%
发文量
102
审稿时长
6 months
期刊介绍:
Russian Journal of Electrochemistry is a journal that covers all aspects of research in modern electrochemistry. The journal welcomes submissions in English or Russian regardless of country and nationality of authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


