Endothelial bionic-modified anti-thrombotic PMP hollow fiber membranes for dual-circulation artificial uterus system
IF 9.5
引用次数: 0
Abstract
The Artificial Womb Technology (AWT) system is a piece of biomedical equipment that supports the in vitro development of extremely premature infants. The system draws fetal blood, oxygenates it, removes carbon dioxide (CO2), and then delivers it back to the fetus. This prevents the fetus from switching to a pulmonary breathing pattern prematurely, which provides critical time for lung tissue development. Researchers have utilized extracorporeal membrane oxygenation (ECMO) technology to provide the fetus with oxygen. In this study, we developed a new method using artificial blood instead of maternal blood in a liquid-liquid dual-circulation. Additionally, since preterm infants require greater blood compatibility and the oxygenated membrane must have anticoagulant properties, the membrane was modified to enhance hemocompatibility and anticoagulant properties. PMP membranes were functionalized with polydopamine (PDA), after which (3-(methacrylamido) propyl) dimethyl (3-thiopropyl) ammonium hydroxide inner salt (SPP) and fondaparinux sodium were successively grafted. Protein adsorption reached 18.3 μg/cm2 (64.3 % reduction), while hemolysis rate dropped to 0.19 % (85.4 % reduction). The results confirm that the functionalized modified membrane not only meets the blood compatibility requirements of the dual-circulation system but also accurately replicates the recurrent process of fetal-maternal gas exchange through its biomimetic design, providing key technical support for the clinical translation of the AWT system.
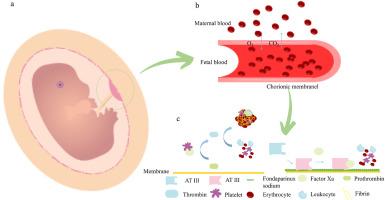
双循环人工子宫系统用内皮仿生改性抗血栓PMP中空纤维膜
人工子宫技术(AWT)系统是一种支持极早产儿体外发育的生物医学设备。该系统抽取胎儿血液,为其充氧,去除二氧化碳,然后将其输送回胎儿体内。这可以防止胎儿过早地切换到肺呼吸模式,为肺组织发育提供关键时间。研究人员利用体外膜氧合(ECMO)技术为胎儿提供氧气。在这项研究中,我们开发了一种在液-液双循环中使用人工血液代替母体血液的新方法。此外,由于早产儿需要更高的血液相容性,而氧合膜必须具有抗凝血性能,因此对膜进行了修饰以增强血液相容性和抗凝血性能。用聚多巴胺(PDA)对PMP膜进行功能化处理,然后依次接枝(3-(甲基丙烯酰胺)丙基)二甲基(3-硫丙基)氢氧化铵内盐(SPP)和氟达哌啶钠。蛋白质吸附达到18.3 μg/cm2(降低64.3%),溶血率降至0.19%(降低85.4%)。结果证实,功能化修饰膜不仅满足了双循环系统的血液相容性要求,而且通过仿生设计准确复制了胎母气体交换的循环过程,为AWT系统的临床转译提供了关键技术支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


