An alternative to in-vivo/in-vitro drug toxicity testing: Environmentally relevant approaches for toxicity assessment of pharmaceuticals towards rats
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
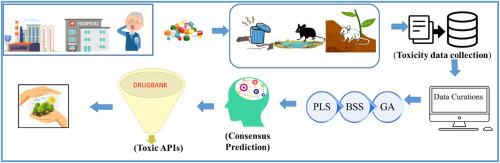
Excessive and inappropriate usage of human and veterinary pharmaceuticals alongside their improper disposal give rise to the contaminants of emerging concern (CEC), potentially harming the non-target living organisms. To meet European Medicines Agency (EMA) guidelines, comprehensive toxicological information is required for each chemical before market release. In this regard, quantitative structure-toxicity relationships (QSTRs) offer effective alternatives. This study aims to develop QSTR models based on the oral median lethal dose (LD50) values of 702 pharmaceuticals in rats, adhering to the OECD principles. Several globally accepted validation parameters were analyzed to establish the reliability, robustness, etc., of the developed QSTR models. Intelligent consensus prediction (ICP) was applied to the individual models to boost predictivity of the models. This study elucidated that electronegativity, lipophilicity, the presence of pyrroles, and the number of tertiary amine groups are responsible for the toxicity toward rats. Further, the DrugBank database (investigational and experimental groups) was screened by the optimal model and the results were validated using real-world toxicity data. The developed models will greatly reduce the cost, time, and animal experimentation in the context of pharmaceuticals-induced toxicity in rat models, enabling toxicity data gap bridging and the development of safe pharmaceuticals strictly supporting RRR (reduction, refinement, and replacement) guidelines.
体内/体外药物毒性试验的替代方法:对大鼠进行药物毒性评估的环境相关方法
人类和兽药的过度和不当使用以及处置不当会产生新出现的关注(CEC)污染物,潜在地伤害非目标生物。为了符合欧洲药品管理局(EMA)的指导方针,每种化学品在上市前都需要全面的毒理学信息。在这方面,定量结构-毒性关系(qstr)提供了有效的替代方法。本研究旨在遵循OECD原则,建立基于702种药物大鼠口服致死中位剂量(LD50)值的QSTR模型。分析了几个全球公认的验证参数,以建立所开发的QSTR模型的可靠性、鲁棒性等。将智能共识预测(ICP)应用于个体模型,提高模型的预测能力。本研究阐明了电负性、亲脂性、吡咯的存在和叔胺的数量是对大鼠毒性的主要原因。进一步,通过优化模型筛选DrugBank数据库(研究组和实验组),并使用实际毒性数据验证结果。开发的模型将大大减少药物诱导毒性大鼠模型的成本、时间和动物实验,使毒性数据弥合差距和开发严格支持RRR(减少、改进和替代)指南的安全药物成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


